According to the SVGO Recommendations 2020, the choice of initial osteoporosis therapy depends largely on the fracture risk [1]. How the sequence of potent antiresorptive therapies can be optimized for patients at high risk of fracture is now demonstrated by recent Swiss real-world data [2]. Study director Prof. Peter Burckhardt explains the highlights of the retrospective analysis in the video.
It is estimated that one in three women aged 50 years and older will experience an osteoporotic fracture, making osteoporosis the most common skeletal disease [3]. To counteract the occurrence of fractures and the often serious health, social, and financial consequences associated with them, women at increased risk of fracture should be treated with medication according to the current recommendations of the Swiss Association Against Osteoporosis (SVGO) [1, 3]. Which drug is used initially depends primarily on the individual fracture risk [1].
Long-term use of denosumab in high fracture risk.
If the risk of fracture is high, the SVGO recommends antiresorptive therapy with denosumab (Prolia®), among others [1]. The monoclonal antibody decreases bone resorption and increases bone mass and strength, thus counteracting the occurrence of fractures [4]. In the randomized phase III FREEDOM trial of 7,808 postmenopausal women with osteoporosis, three years of denosumab treatment reduced the risk of vertebral fractures by 68% compared with placebo (p<0.001). The risk of hip fracture decreased by 40% (p=0.04) in the overall population [5] and by as much as 62% (p=0.007) in women older than 75 years [6]. That long-term treatment with denosumab of up to ten years can further reduce the risk of non-vertebral fractures compared to three years of treatment with good tolerability is shown by the results of the FREEDOM extension [7]. If a change in therapy is considered despite the recommended long-term use of denosumab, it should be kept in mind that the beneficial effects of denosumab are reversible and that the loss of bone mineral density (BMD) gained under denosumab is associated with a renewed increase in fracture risk [2].
Retrospective analysis examines incidence of fractures after denosumab
A Swiss-wide retrospective study (Investigator-sponsored Study [ISS]) now investigated, using data from 797 women, which factors may promote or prevent the occurrence of new vertebral fractures following denosumab. As the results of the FREEDOM study had already shown, vertebral body fractures also occurred significantly less frequently in the real-world setting during treatment with denosumab. If patients did not receive denosumab, i.e., in the periods before and after treatment, fracture incidence was increased (Figure 1) [2].
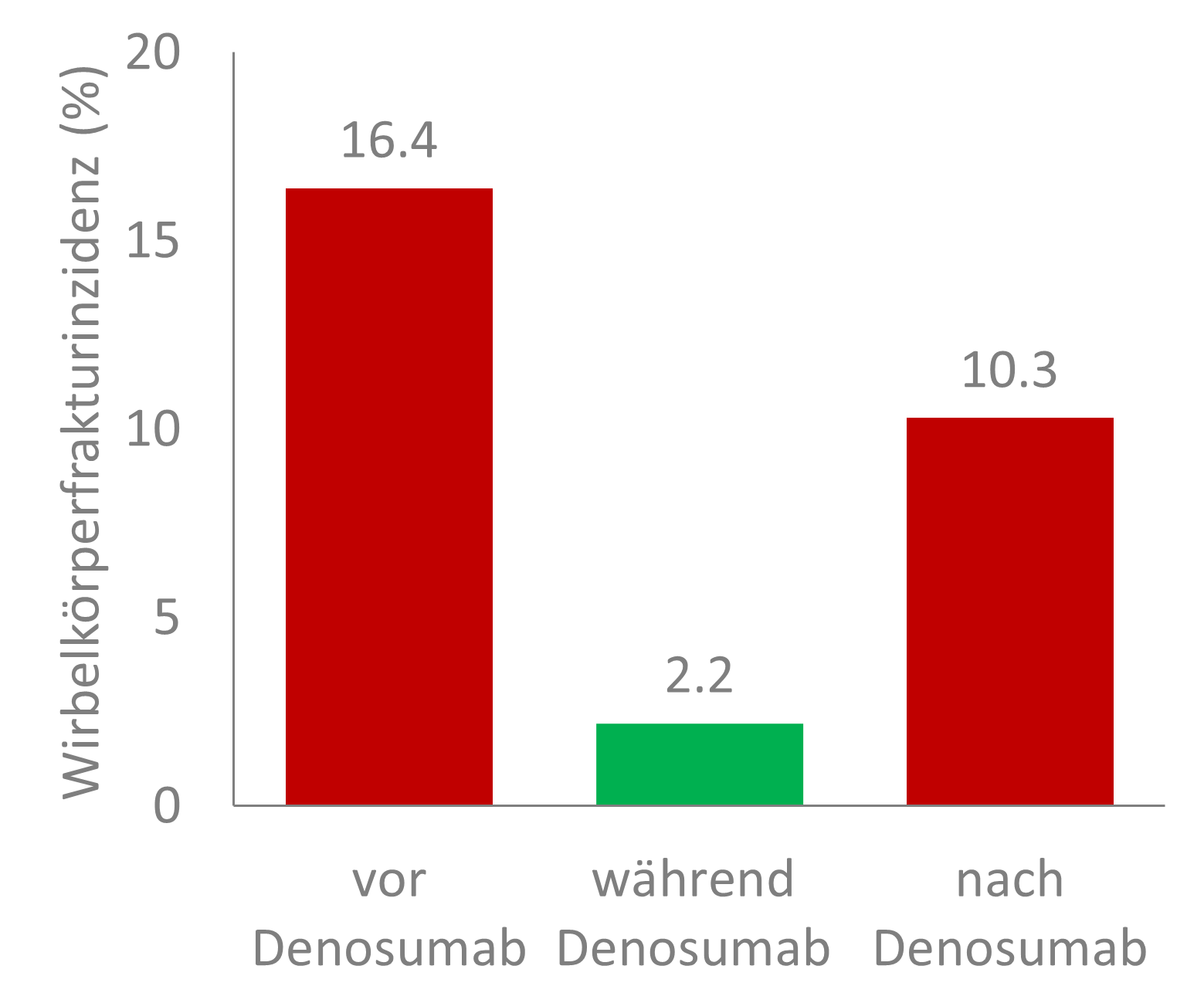
Figure 1: Incidence of vertebral fractures before, during, and after treatment with denosumab in a nationwide retrospective study of 797 postmenopausal women with osteoporosis or women with breast cancer without metastases undergoing adjuvant therapy with aromatase inhibitors who had received at least two injections of denosumab and completed treatment with a follow-up of at least one year. Adapted from [2].
Which factors prevent and which promote vertebral fractures after denosumab?
Using questionnaires covering the periods before, during, and after denosumab, the following protective and risk factors for the occurrence of vertebral fractures following denosumab were identified (Table 1) [2]:
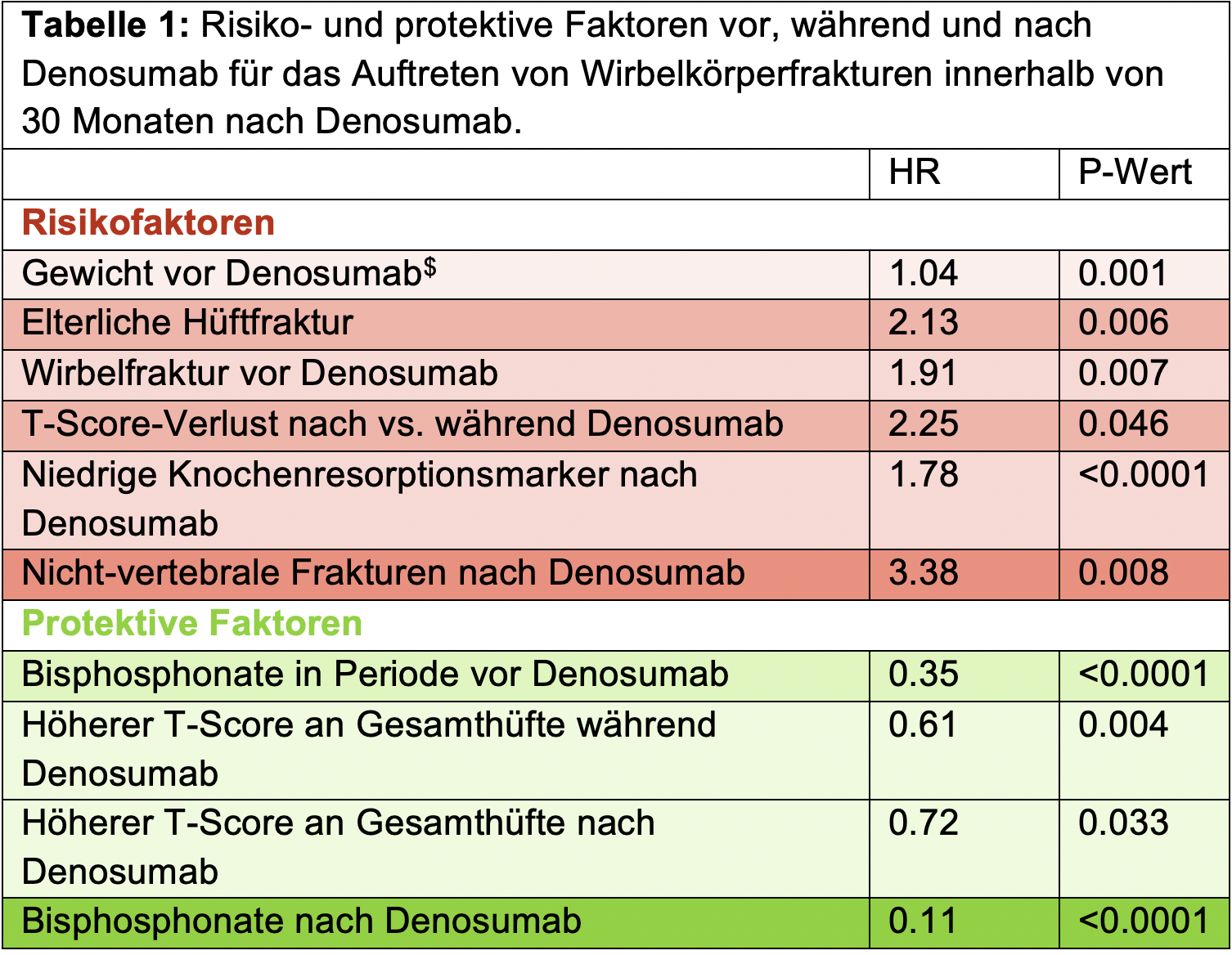
$ Multivariate Analysis. All others: Univariate analysis. HR: Hazard Ratio. Non-statistically significant factors are not shown and can be viewed in the original publication. Adapted from [2].
Protective effect of bisphosphonate follow-up therapy not enhanced by additional bisphosphonate pretherapy
Of all the factors studied, the use of bisphosphonates after denosumab proved to have the strongest protective effect (Table 1) [2]. Thus, vertebral body fractures occurred in 2.9% of patients with follow-up bisphosphonate therapy following denosumab – without treatment with bisphosphonates, this was the case in 36.4% of patients (Figure 2). Although bisphosphonate treatment before denosumab also reduced the risk – 12.4% of patients with such treatment suffered vertebral fractures following denosumab. However, the use of bisphosphonates before denosumab had no additional protective effect when patients also received bisphosphonates after denosumab [2]. This confirms the first-line use of denosumab in postmenopausal osteoporosis patients at high risk of fracture [4].
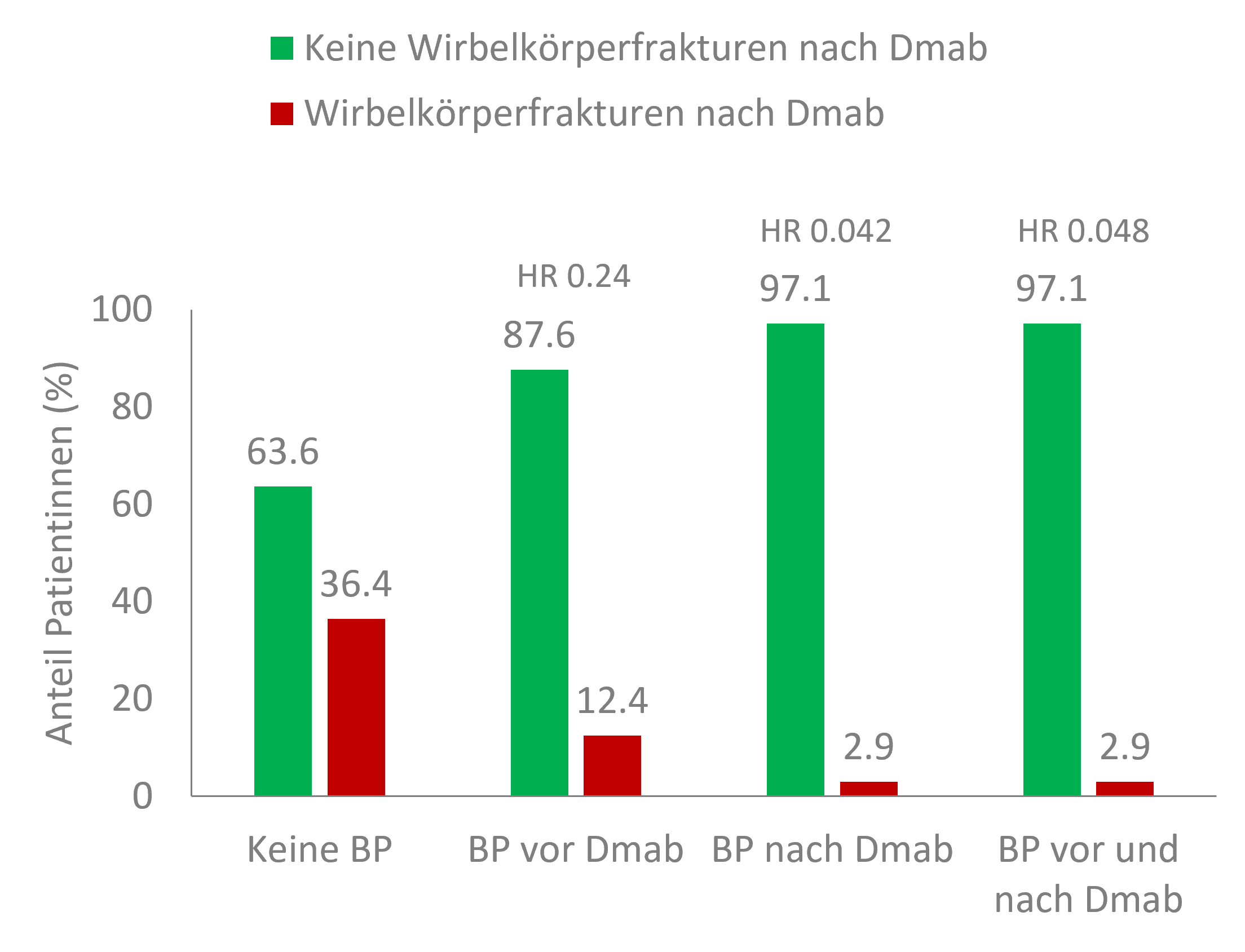
Figure 2: Protective effect of bisphosphonate treatment before and after denosumab related to the incidence of vertebral fractures following denosumab in a nationwide retrospective study. BP: bisphosphonates; Dmab: denosumab. Adapted from [2].
Conclusion
According to the results of the phase III FREEDOM study and its extension, treatment with denosumab (Prolia®) can reduce the osteoporotic fracture risk in postmenopausal women in the long term and is recommended by the SVGO as initial therapy for high fracture risk [1, 7, 8]. As the results of the current Swiss retrospective study show, the monoclonal antibody significantly reduces fracture risk in postmenopausal women* even in the real-world setting. In addition, the finding that the incidence of vertebral fractures is kept low by post-treatment bisphosphonates even in the case of a change in therapy, without the need for pre-treatment bisphosphonates, may help optimize sequential therapy management and supports the use of denosumab in the first line of treatment in postmenopausal women at high risk of fracture [2].
* The focus is on patients with osteoporosis, although women with breast cancer without metastases on adjuvant therapy with aromatase inhibitors were also included.
Highlights of the retrospective study published in the Journal of Bone and Mineral Research by Principal Investigator Prof. Peter Burckhardt, Clinic Bois Cerf/Hirslanden, Lausanne, can be viewed in the following video:
Want to learn more about SVGO Recommendations 2020?
Click here for a descriptive summary in text and video form!
This text was produced with the financial support of Amgen Switzerland AG, Rotkreuz.
CH-PRO-0821-00001-E
Brief technical information Prolia®
Article online since 23.08.2021
Literature