Currently, gemcitabine plus Nab-paclitaxel and FOLFIRINOX are considered standard first-line therapies for advanced pancreatic cancer. So far, there is no direct comparison of these options. An analysis of the German tumor registry shows: Prognosis remains poor regardless of treatment choice, but in the high-risk group, treatment choice appears to have a significant impact on survival.
With a 5-year survival rate of only 8%, inoperable or metastatic pancreatic cancer is a dangerous disease for which no satisfactory treatment option is available to date. Due to the initial often unspecific and discrete symptoms, as well as the rapid progression, about 80% of all newly diagnosed tumors are already so advanced that resection is not an option. In these cases, systemic therapy with gemcitabine was previously considered the standard of care. It has now been shown that the additional administration of nab-paclitaxel – i.e. the albumin-based formulation of paclitaxel – significantly increases the remission rate and prolongs survival by an average of almost two months compared to monotherapy [1]. Alternatively, FOLFIRINOX, a combination of fluorouracil, folinic acid, irinotecan, and oxaliplatin, is used for first-line treatment of unresectable pancreatic cancer. In previous studies, this therapy increased remission rates and prolonged progression-free survival by an average of 3.1 months compared with gemcitabine alone in patients in good general health. However, the rate of serious adverse events was significantly higher with FOLFIRINOX in studies [1]. Besides FOLFIRINOX and gemcitabine/nab-paclitaxel, erlotinib is currently the only combination agent approved for first-line therapy [1].
The agony of choice
In the context of two treatment options with different toxicity profiles and only minor variation in overall survival, treatment selection is challenging. The question of the transferability of data collected in studies to the actual patient population also plays a role here. This is because, as is well known, the latter is often older and sicker than the study population. Therefore, data outside of clinical trials are needed to evaluate the available drugs. Using registry data collected over a large area and over a longer period of time, the effectiveness of treatments can be assessed in a more differentiated manner and thus the quality of care in everyday life can be improved. This is particularly necessary when – as in advanced pancreatic cancer – studies directly comparing therapeutic approaches are lacking.
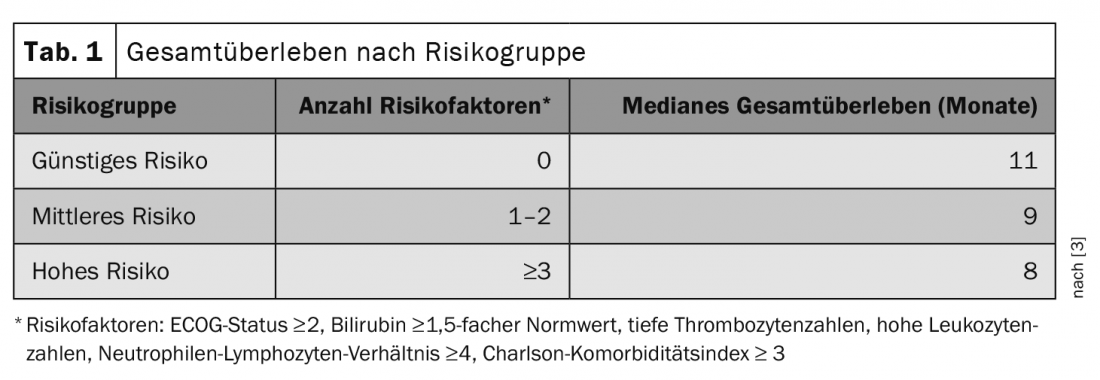
By analyzing data from the German Pancreatic Cancer (TPK) tumor registry, a prognostic score for patients with metastatic pancreatic cancer could be developed in 2020 and the two established first-line treatment strategies could be compared [2,3]. In total, the evaluation included over 1730 patients. 443 of these received gemcitabine/nab-paclitaxel and 255 received FOLFIRINOX. In the identified prognostic groups, researchers compared the efficacy of the two first-line therapies. Risk factors included an ECOG (Eastern Cooperative Oncology Group) status ≥ 2, bilirubin elevation above 1.5 times normal, low platelet counts, high leukocyte counts, a neutrophil-to-lymphocyte ratio ≥4, and a Charlson comorbidity index ≥3. An increasing number of existing risk factors was associated with shorter overall survival (Table 1) [3].
The worse the prognosis, the more important the choice of therapy
While treatment choice was not reflected in overall survival in more prognostically favorable situations, patients with at least three risk factors benefited significantly from therapy with FOLFIRINOX. In this high-risk group, the median survival with gemcitabine/nab-paclitaxel treatment was 4.3 months and that with FOLFIRINOX therapy was 10.7 months [3].
Although the side effect profile and individual factors must not be disregarded, treatment with FOLFIRINOX appears to confer benefits in the most prognostically unfavorable situations. Wider adoption of a risk score for treatment selection and validation of these results in further analyses could potentially simplify the management of unresectable pancreatic cancer in the future.
Literature:
- Oettle H, et al: Pancreatic cancer – Onkopedia. October 2018. www.onkopedia.com/de/onkopedia/guidelines/pankreaskarzinom/@@guideline/html/index.html (last accessed Jan. 24, 2021).
- Hegewisch-Becker S, et al: Results from the prospective German TPK clinical cohort study: Treatment algorithms and survival of 1,174 patients with locally advanced, inoperable, or metastatic pancreatic ductal adenocarcinoma. Int J Cancer. 2019; 144(5): 981-990.
- Hegewisch-Becker S, et al: Prognosis in patients with advanced pancreatic cancer and effectiveness of1st-line treatments – a prognostic and comparative effectiveness study using propensity score weighting for patients treated with Gem+Nab vs. FOLFIRINOX from the TPK registry. Abstract presented at the Annual Meeting of the German, Austrian, and Swiss Societies of Hematology and Medical Oncology; virtual conduct, Oct 09-11, 2020.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(1): 21.