In addition to improving glycemic control, GLP-1 receptor agonists have proven additional cardiovascular benefits. Large endpoint studies have demonstrated reductions in myocardial infarction and stroke rates and cardiovascular mortality. Another important cardioprotective factor is the beneficial effects on weight control. In addition, there are impressive new findings from a large study program on semaglutide.
In addition to lowering HbA1c, GLP-1 receptor agonists (GLP-1-RA) in the vasculature lead to improved endothelial function and a reduction in atherosclerosis. In addition, other beneficial effects, including inhibition of peripheral inflammation, have been demonstrated. “It is probably the multiplicity of these effects that explains the cardioprotection with GLP-1 receptor agonists,” summarized Prof. Juris Meier, MD, Chief of Diabetology, Katholisches Klinikum Bochum (D), at this year’s virtual edition of the Diabetologie grenzenlos congress [1]. Reductions in myocardial infarction and stroke rates, as well as cardiovascular mortality, have been demonstrated repeatedly with treatment with GLP-1-RA. In contrast to SGLT-2 inhibitors, no effects on heart failure could be detected. With regard to renal endpoints, an improvement in albuminuria, i.e., a surrogate parameter of renal function, was shown, but there is no evidence yet for a longer-term stabilization of glomerular filtration rate (GFR), the speaker said.
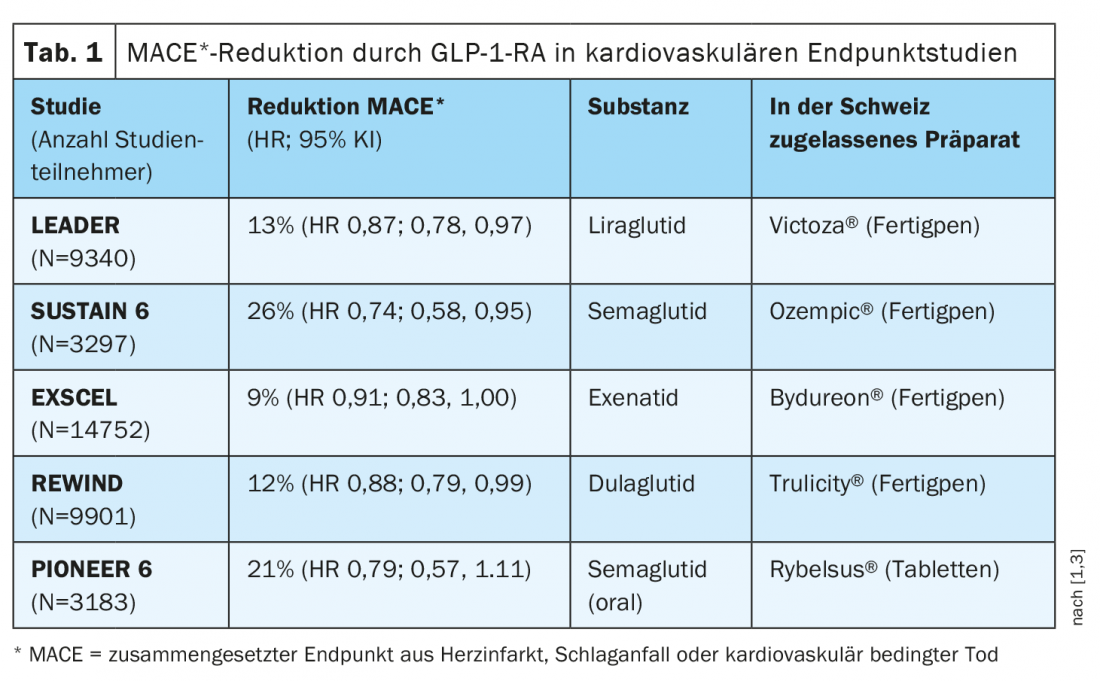
Cardiovascular added benefit of GLP-1-RA is empirically proven.
In several cardiovascular outcomes studies, GLP-1 RA significantly reduced the composite endpoint of myocardial infarction, stroke, or cardiovascular-related death (“MACE”) (Table 1). Results from the REWIND trial published in the Lancet in 2019 showed a significant 12% reduction in MACE in the dulaglutide group compared to placebo [2]. The median follow-up period was 5.4 years, which is the longest follow-up period in the GLP-1 receptor agonist class. “I believe that the strong HbA1c reduction plays a very important role in the cardiovascular event reduction by the GLP-1 receptor agonists,” the speaker explained. Even though intervention studies have so far had little success in directly demonstrating this link, he said, it is epidemiologically clear that elevated HbA1c levels are associated with a higher cardiovascular event rate.
HbA1c reduction is definitely important
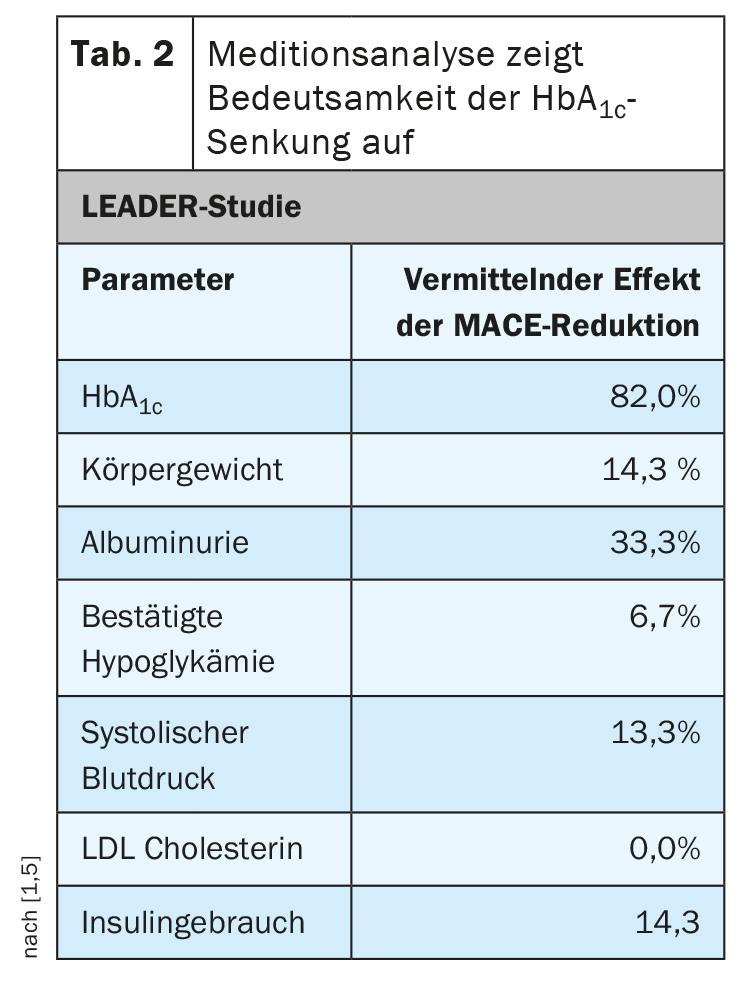
In particular, the long-acting GLP-1 receptor agonists lead to significant HbA1c reduction and this is an important factor, according to Prof. Meier [1]. “With all the euphoria for compound-specific cardiovascular effects, let’s please not forget the importance of lowering blood glucose,” the diabetologist stresses. In a recent meta-analysis of data from the large endpoint trials in which Prof. Meier and colleagues examined the effects of GLP-1-RA, DPP-4 inhibitors, and SGLT-2 inhibitors, they found a linear relationship between HbA1c reduction and cardiovascular event rates. “The more HbA1c reduction, the fewer cardiovascular events,” the speaker summarized. This association is particularly clear in the case of strokes, he said. One finds evidence of such associations not only when analyzing the data of all endpoint studies, but also when looking at the results of individual studies. “This has been shown particularly impressively in the LEADER study with liraglutide,” Prof. Meier reports. A mediation analysis was performed to investigate the relationship between the effect of liraglutide on certain parameters and cardiovascular event reduction. It became clear that the decisive factor was the HbA1c reduction, which could explain 82% of the effects (Tab. 2) [1,4].
Weight reduction is promoted – new study on semaglutide
Overweight and obesity are also known to be important cardiovascular risk factors. The fact that GLP-1-RA, in addition to lowering HbA1c levels, reducing systolic blood pressure and reducing LDL cholesterol, also has a particularly positive effect on weight control has already been demonstrated several times. The most recent evidence on this comes from a phase III multicenter trial program. In the STEP-1 study, 1961 adults with significant obesity (BMI ≥30 kg/m2) were studied in a total of 16 countries in Asia, Europe, North America, and South America. Study participants received semaglutide (Ozempic®) 2.4 mg or placebo once weekly, each supplemented with lifestyle counseling regarding diet and exercise. At 68 weeks after baseline, weight loss was 14.9% in the verum group and 2.4% on placebo. This corresponds to an average decrease in body weight of 15.3 kg vs. 2.6 kg. Under treatment with semaglutide, more than one-third of the subjects managed to lose more than 20% of their body weight by the end of the study, and more than 80% of the participants achieved a 5% weight loss compared to baseline. In the placebo condition, this proportion was only 31.5%. Weight loss of at least 10% was achieved with semaglutide by 69.1% compared with 12.0% with placebo. The most common side effects were nausea and diarrhea; in general, these were mild to moderate and decreased over the course of treatment. 4.5% of participants on semaglutide and 0.8% on placebo discontinued the study early due to adverse events. The STEP-3 study confirmed the large weight losses with semaglutide [6]. 611 obese individuals with a mean baseline weight of approximately 106 kg (233 pounds) and a BMI of 38 kg/m2 at 41 sites in the United States participated in the study and received semaglutide 2.4 mg or placebo once weekly for 68 weeks, each in combination with an intensified diet and exercise program. At 68 weeks after baseline, weight loss in the semaglutide group averaged 16% of baseline body weight, equivalent to just under 17 kg. In the placebo condition, weight loss was 5.7% or 6.4 kg. In summary, the STEP-3 study confirmed that semaglutide promotes weight loss and also showed that these effects can be further enhanced by intensifying lifestyle modification with regard to diet and exercise.
In addition to the subcutaneously injected preparation Ozempic®, Rybelsus® is also available as an oral formulation of the active ingredient semaglutide [3].
Source: Diabetology without borders 2021
Literature:
- Meier J: Cardiovascular risk management of type 2 diabetes mellitus: cardiovascular and renal endpoints-an update 2021. GLP-1 agonists. Prof. Juris Meier, MD. Diabetology Limitless, Feb. 26, 2021.
- Gerstein HC, et al: Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet 2019; 394(10193): 121-130.
- Swiss Drug Compendium, https://compendium.ch, (last accessed Feb. 27, 2021).
- Wilding JPH et al: Once-Weekly Semaglutide in Adults with Overweight or Obesity. Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine 2021, DOI: 10.1056/NEJMoa2032183.
- Marso SP, et al: LEADER Trial: liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
- Wadden TA, et al: Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity. The STEP 3 Randomized Clinical Trial. JAMA. Published online February 24, 2021. doi:10.1001/jama.2021.1831
HAUSARZT PRAXIS 2021; 16(3): 26-28 (published 10/3/21, ahead of print).











