Purpose: Does immunomodulatory therapy with rituximab (Mabthera®) lead to sustained symptom improvement in patients suffering from chronic fatigue syndrome (CFS)?
Background: In CFS an agonizing psychophysical exhaustion up to immobilization and invalidity is found, which is clinically different from “normal fatigue” or even depression. The prevalence is likely to be around 0.3%. In accordance with the enormous pressure of suffering, many things were tried, unfortunately with little success.
The immune system seems “flu-like” overactivated, so appropriate modulation might do some good. In this study, an approach using depletion of CD20 lymphocytes by the monoclonal antibody rituximab (approved for NH lymphoma, rheumatoid arthritis, and vasculitis) is being pursued.
Patients and Methods: 29 patients with CFS (according to Fukuda criteria) of varying severity received rituximab infusions in this Norwegian open-label phase II study at doses commonly used, for example, in the treatment of rheumatoid arthritis. This was repeated after 2 weeks and at 3, 6, 10, and 15 months, with follow-up up to 24 months, and in 19 patients also up to 36 months after initial dose.
Every 2 weeks, the symptom burden according to self-assessment of “fatigue” (from 0-10) and, secondarily, the SF-36 score, which asks about the health situation in 8 domains (such as “vitality” and “social functioning”), were used as outcomes.
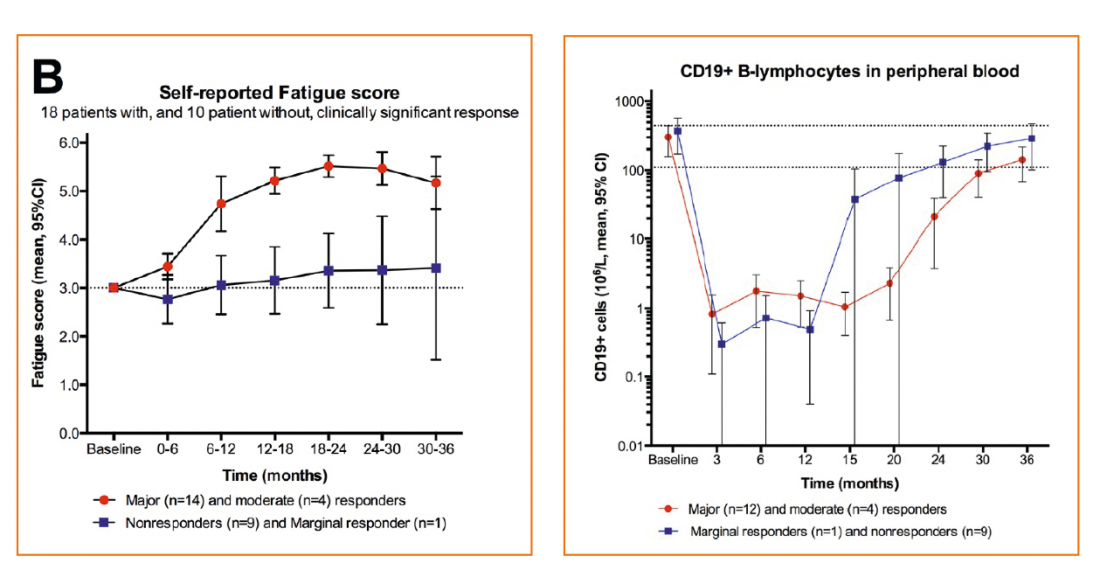
Response was defined as a fatigue score of ≥4.5 over 6 weeks with intermittent score ≥5.
Results: Response criteria were achieved in 18 of 29 patients and then mostly sustained. Interestingly, this occurred at the earliest after three months and correlated with suppression of CD20 lymphocytes. SF36 results also showed highly relevant improvements.
No serious side effects occurred.
Authors’ conclusions: The authors see their earlier pilot study confirmed and suggest that many CFS sufferers may benefit impressively from rituximab or CD20 depletion and have consequently initiated a double-blind placebo-controlled phase III study (with 152 patients).
Also, the results supported immunological factors in the pathogenesis of the disease.
Comment: This study may seem very special at first, but in fact it has rightly met with a great response from experts and those affected. This is because the immunological approach is plausible and the result comparatively outstanding. The open design and the manageable number of cases remain to be criticized.
In response to the results of the previous pilot study, ex-professional footballer Olaf Bodden, who suffers from CFS, organized off-label treatment with this regimen in 2013 – but subsequently experienced a deterioration. This anecdote shows that euphoria is premature, but still marks a real hope in the bleak CFS situation and now needs to be further evaluated. The Norwegians have collected donations for the follow-up study, and the price for an infusion (2 ampoules) with the Roche product is 3751.60 CHF, as no cheaper product is yet available despite patent expiry.
InFo NEUROLOGY & PSYCHIATRY 2016; 14(6): 41.











