Inhaled corticosteroids (ICS) are used regularly in COPD patients . It is well known that this is associated with an increased risk of pneumonia. However, what about the risk of Pseudomonas aeruginosa infection? Danish scientists investigated this question.
The research group led by Dr. Josefin Eklöf from the Department of Internal Medicine, Section of Respiratory Medicine, Herlev and Gentofte Hospital, University of Copenhagen, included a total of 21,408 Danish COPD patients in their multiregional cohort study [1], of whom 763 (3.6%) experienced Pseudomonas aeruginosa infectionduring follow-up.
Previous observational studies have already shown that the presence of a positive respiratory culture with Pseudomonas aeruginosa in patients with COPD is associated with severe disease, frequent hospitalizations, and increased mortality. Accordingly, this subgroup of COPD patients may be particularly vulnerable to the potential adverse effects of pharmacotherapy with ICS. However, few data were available on ICS as a risk factor for P. aeruginosa, and a possible association between ICS use and the risk of P. aeruginosa in patients with COPD has not been investigated.
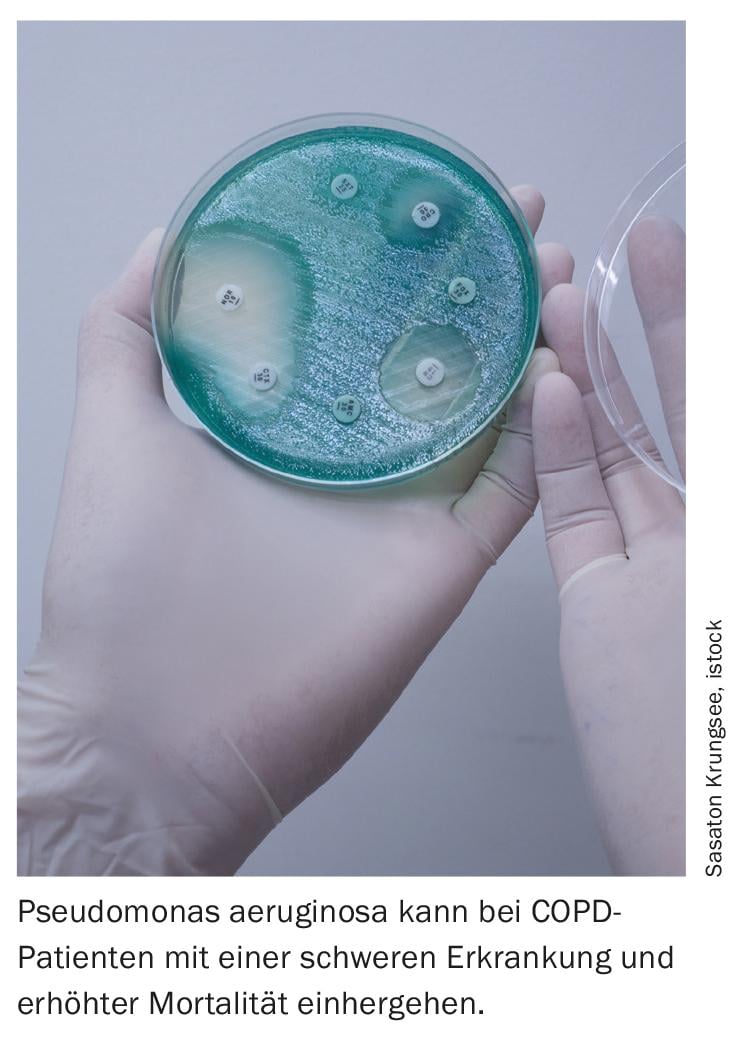
The higher the dose, the greater the risk
The Danish study now considered patients who had registered for an outpatient clinic visit in the Danish COPD registry DrCOPD between 2010 and 2017. All prescriptions for ICS, alone or in the combination inhaler, filled 365 days before cohort entry were identified; ICS included beclomethasone, budesonide, fluticasone, ciclesonide, and mometasone.
All doses of ICS were converted to budesonide-equivalent doses: Beclometasone and mometasone were considered budesonide equivalent. Fluticasone propionate and ciclesonide were converted to budesonide doses in a ratio of 2:1 and 2.5:1, respectively. ICS doses were categorized by dividing ICS exposure into low (<400 μg), medium (400-800 μg), and high (>800 μg) daily doses (according to international GINA guidelines). Non-use during the entire period was used as a reference.
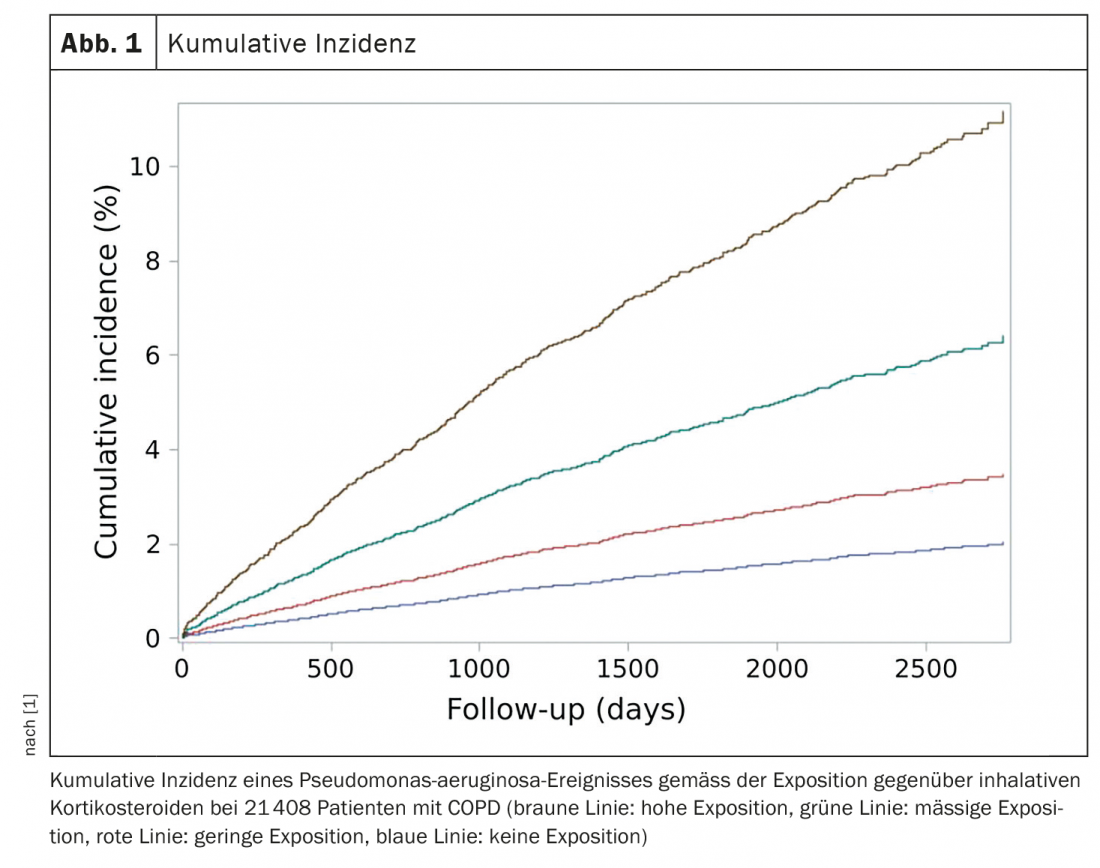
In their data analysis, the researchers concluded that ICS use was associated with a significantly increased risk of P. aeruginosa compared with non-use. In patients with the highest ICS exposure, there was a strong dose-dependent 3.5-fold increased risk of P. aeruginosa. There was a strong association between ICS dose and outcome, with P. aeruginosa risk increasing with ICS exposure in a dose-dependent manner: at a low ICS dose, the hazard ratio (HR) was 1.38 (95% CI 1.03-1.84, p=0.03); at a moderate dose, the HR was 2.16 (95% CI 1.63-2.85, p<0,0001). Patients with the highest ICS exposure had a 3.6-fold increased risk (HR 3.58, 95% CI 2.75-4.65, p<0.0001) (Fig. 1) .
The use of ICS in COPD patients was associated with a significantly increased and dose-dependent risk of P. aeruginosa acquisition, the authors concluded. Therefore, they advise caution when administering high doses of ICS in critically ill patients with COPD. Other settings in comparable cohorts would be advisable in the future to confirm the results.
Literature:
- Eklöf J, Ingebrigtsen TS, Sørensen R, et al: Use of inhaled corticosteroids and risk of acquiring Pseudomonas aeruginosa in patients with chronic obstructive pulmonary disease. Thorax 2021 (online first); doi: 10.1136/thoraxjnl-2021-217160.
InFo PNEUMOLOGY & ALLERGOLOGY 2022; 4(1): 5.
HAUSARZT PRAXIS 2022; 17(3): 52