Mineralocorticoid receptor antagonists are indicated in all stages of chronic heart failure symptomatic with beta-blockade and ACE inhibitor therapy. This is based on the EMPHASIS-HF study. Patients with heart failure NYHA II-IV, an EF <35%, and sinus rhythm with a heart rate >70/min on complete heart failure drug therapy should be treated with the If-channel inhibitor ivabradine. This is based on the SHIFT study. The RAFT trial justifies the now expanded indication for CRT therapy in patients with sinus rhythm and a ventricular complex width of >130 ms with left bundle branch block configuration. In acute heart failure, the RELAX-AHF study with the relaxin derivative Serelaxin has shown interesting results.
In June 2012, the European Society of Cardiology (ESC) presented the new European guidelines on acute and chronic heart failure [1]. This brief review summarizes the guidelines as well as the underlying studies.
Chronic heart failure with reduced ejection fraction
Major therapeutic goals of heart failure therapy are to improve symptoms and prognosis and to decrease hospitalizations [2]. In chronic heart failure with an ejection fraction (EF) <40%, the guideline recommends the use of ACE inhibitors first, followed by beta-blockers [1]. Angiotensin (AT) 1 receptor antagonists are indicated only when an ACE inhibitor is not tolerated [1]. Additive administration of an AT1 antagonist to existing therapy with ACE inhibitors may reduce morbidity [3]. However, a triple combination with an ACE inhibitor, an AT1 antagonist, and a mineralocorticoid receptor antagonist (MRA) is explicitly discouraged in the guideline because of the increased risk of hyperkalemia [1]. Emphasized the importance of achieving target doses of standard therapeutics for prognosis improvement.
Innovations in therapy
Mineralocorticoid receptor antagonists: With regard to the use of MRA, the recommendations of the guidelines have been significantly expanded. The pre-existing indication for patients with severe clinical heart failure (NYHA IV) was based on the results of the RALES trial [4]. The EMPHASIS-HF trial published in 2011 [5] demonstrated that patients with stable, mildly symptomatic heart failure (NYHA II) with an EF ≤35% also benefit from the administration of the MRA eplerenone (Fig. 1) .
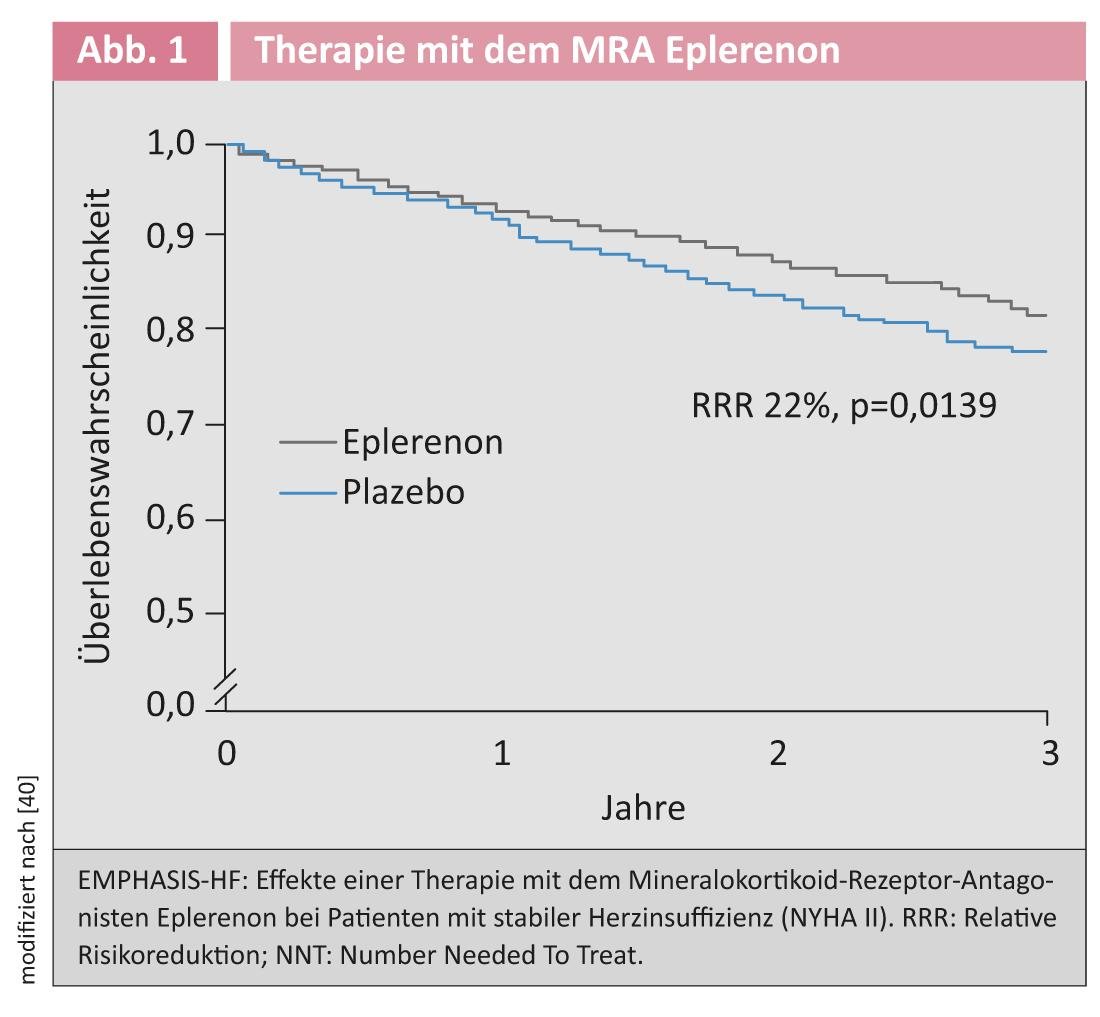
The effect of MRA was tested in the EMPHASIS-HF trial against a background of near-complete ACE inhibitor therapy (93%) and beta blockade (87%). Accordingly, the new guideline recommends treating patients who have symptomatic heart failure with an EF ≤35% despite therapy with ACE inhibitors and beta blockers with an MRA. The results of a recently published meta-analysis indicate that the observed reduction in cardiovascular end points by MRA is due to class effects rather than being attributable to specific differences between individual agents [6]. With this in mind, the guidelines recommend the substance class of MRA, but not the use of a specific agent [1].
If-channel inhibition with ivabradine: increased heart rate is associated with increased mortality in chronic heart failure [7]. Ivabradine leads to selective heart rate reduction via If channel inhibition in the sinus node [7]. An innovation in the guidelines is the recommendation of ivabradine therapy in patients who remain symptomatic with an EF <35% on ACE inhibitors, beta blockers, and MRA and who have sinus rhythm with a heart rate >70/min. This recommendation is based on the results of the SHIFT study. Patients in NYHA stage II-IV with an EF <35% and sinus rhythm with a heart rate >70/min were included [8]. Ivabradine significantly reduced the combined endpoint consisting of cardiovascular death and hospital admissions for heart failure [9] (Fig. 2).
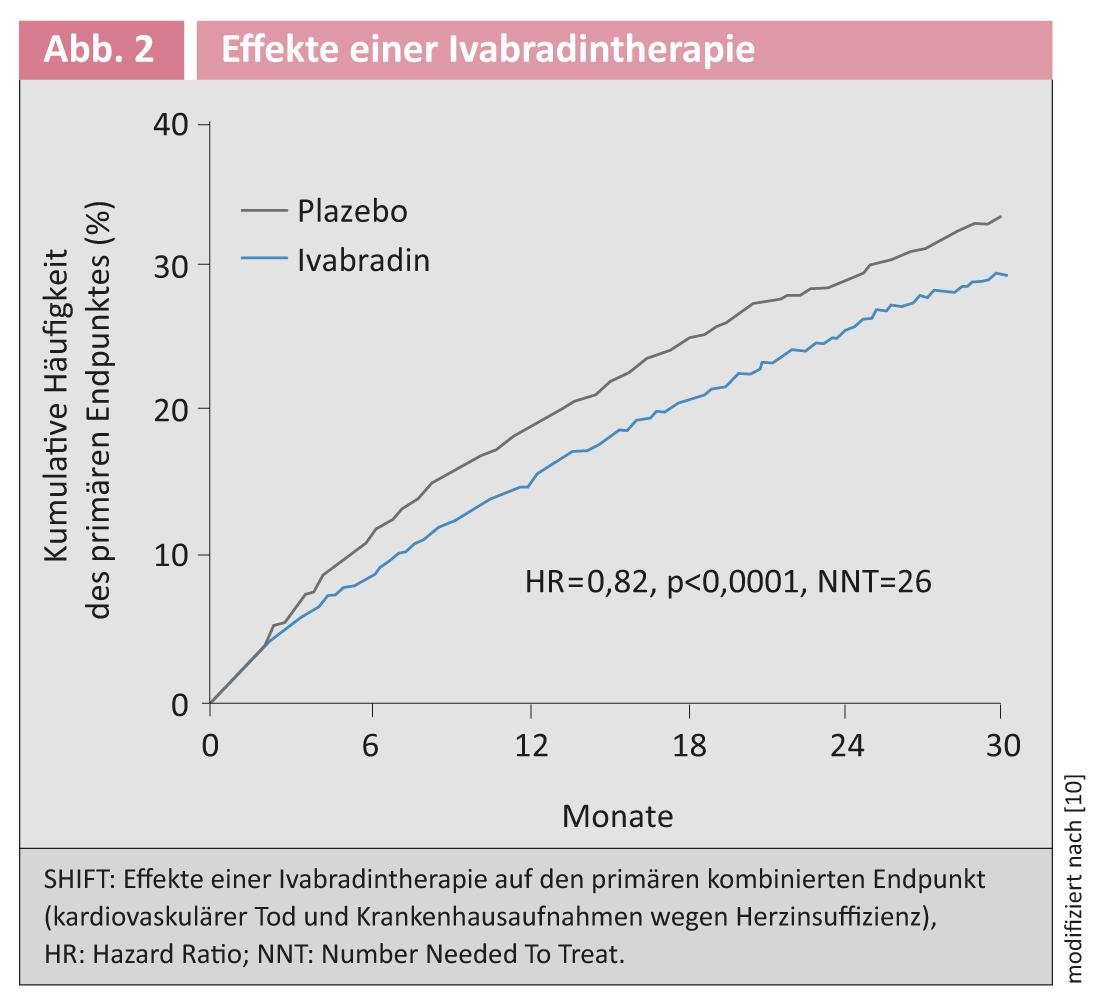
A new analysis showed that not only first hospitalizations, but also second and third hospitalizations were significantly reduced [10]. The guideline recommendations on ivabradine therapy apply against a background of standard drug therapy including maximum tolerated beta-blockade. In the SHIFT trial, more than 85% of patients were treated with a beta-blocker, and the effect of ivabradine was independent of its dose [11]. Also, preexisting therapy with MRA did not affect the observed reduction in the primary end point [12].
Cardiac Resynchronization Therapy: To date, cardiac resynchronization therapy (CRT) with a biventricular pacemaker has been recommended for morbidity and mortality reduction in patients with NYHA stage III-IV, an EF ≤35%, and left bundle branch block with a QRS width ≥120 ms in sinus rhythm [13]. In less symptomatic patients (NYHA II), use was advocated to reduce morbidity. The Resynchronization for Ambulatory Heart Failure Trial (RAFT trial) clarified the open question of whether these less symptomatic patients with stable heart failure (mainly NYHA II) would benefit from receiving a CRT system in addition to implantable cardioverter defibrillator (ICD) therapy [14]. The combined primary end point was shown to decrease by 25% in the ICD/CRT group compared with the ICD alone group (Fig. 3).
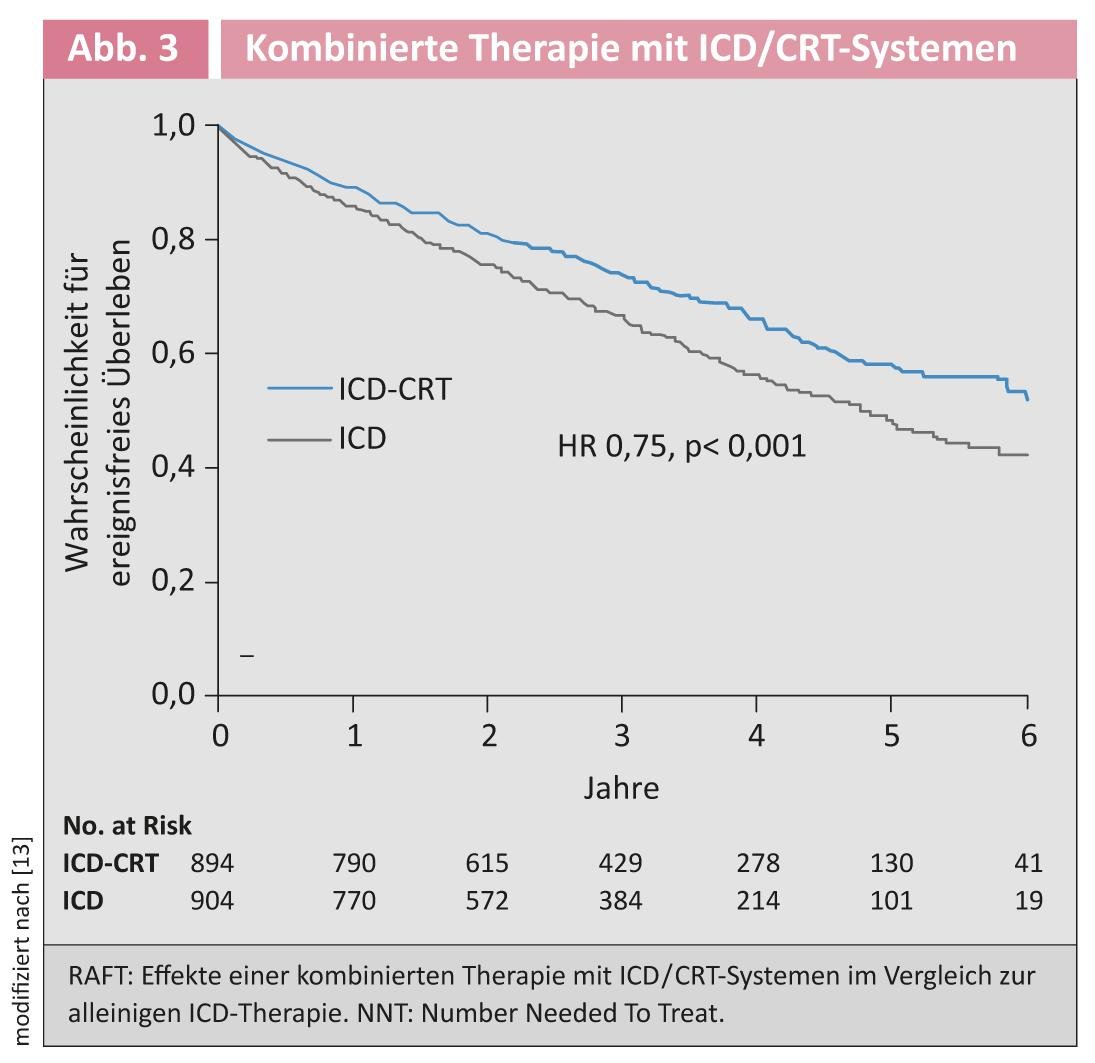
Therefore, the new guidelines recommend implantation of an ICD/CRT system with a class IA recommendation in patients with stable heart failure NYHA II, a QRS duration of >130 ms, and an ejection fraction <30% [1].
Figure 4 provides an overview of treatment options in patients with chronic heart failure.
Acute heart failure
Treatment of acute heart failure is much less evidence-based than that of chronic heart failure. Most recommendations have a recommendation strength of B or C, meaning they rely on small, uncontrolled studies or case collections or expert opinion. Here, recommendations for general measures are made more than specific therapies [15]. Clear class I recommendations are: Administration of diuretics, application of oxygen in hypoxemic patients, thromboembolism prophylaxis. The administration of vasodilators or positive inotropic agents depends on the hemodynamic situation of the patients [1].
Overview of tested therapies
The placebo-controlled trials in acute heart failure listed below have had neutral or negative outcomes and have not contributed to the advancement of acute heart failure therapy.
Calcium sensitizer levosimendan: Lev osimendan sensitizes contractile proteins to calcium and thus has a positive inotropic effect. In addition, it leads to afterload reduction via potassium channel opening. The randomized SURVIVE trial failed to demonstrate a significant reduction in 180-day mortality with levosimendan compared with dobutamine therapy alone [16]. However, a slight improvement in dyspnea symptoms was observed. There was an increase in threatening hypotension during the initial and saturation phases with levosimendan. According to current guidelines, the efficacy and safety of levosimendan therapy is still unclear. Use is recommended with weak evidence (IIbC) in selected patients [17].
PDE inhibitor milrinone: The OPTIME-CHF study evaluated milrinone versus placebo and failed to demonstrate improvement in hard clinical endpoints. However, there was an increase in atrial and ventricular tachyarrhythmias and sustained hypotension. Numerically, there was even an increase in deaths [18].
Adenosine A1 antagonist rolofylline: the PROTECT trial failed to demonstrate a reduction in the combined endpoint with rolofylline versus placebo in patients with acute heart failure and impaired renal function [19]. About 1% of patients experienced a seizure, probably via inhibition of central A1 receptors.
Recombinant BNP nesiritide: The ASCEND-HF study included 7141 patients with acute heart failure. This showed a modest improvement in breathlessness, whereas the clinical endpoints (all-cause mortality or re-hospitalization rate) were not affected. There was an increase in hypotonies [20].
Vasopressin antagonist tolvaptan: The EVEREST trial showed no reduction in mortality or hospitalizations with tolvaptan [21]. Significant side effects were not observed.
New therapies
Derivative of the pregnancy hormone relaxin: The pregnancy hormone relaxin regulates the preparation of the birth canal at the end of pregnancy. It also leads to cardiovascular changes such as an increase in cardiac output, a decrease in peripheral vascular resistance, and an increase in glomerular filtration rate [22]. This spectrum of action makes Relaxin an ideal therapeutic agent for acute heart failure. In the randomized RELAX-AHF trial, 581 patients were treated with the relaxin derivative Serelaxin and 580 with placebo [23]. Here, we examined shortness of breath as the primary end point and survived days out of hospital, as well as cardiovascular death or re-hospitalization for heart failure. Dyspnea symptoms improved significantly in the relaxation group within the first five days. The secondary endpoint was not affected. Interestingly, however, a significant decrease in cardiovascular mortality was observed after 180 days (Fig. 5) [34].
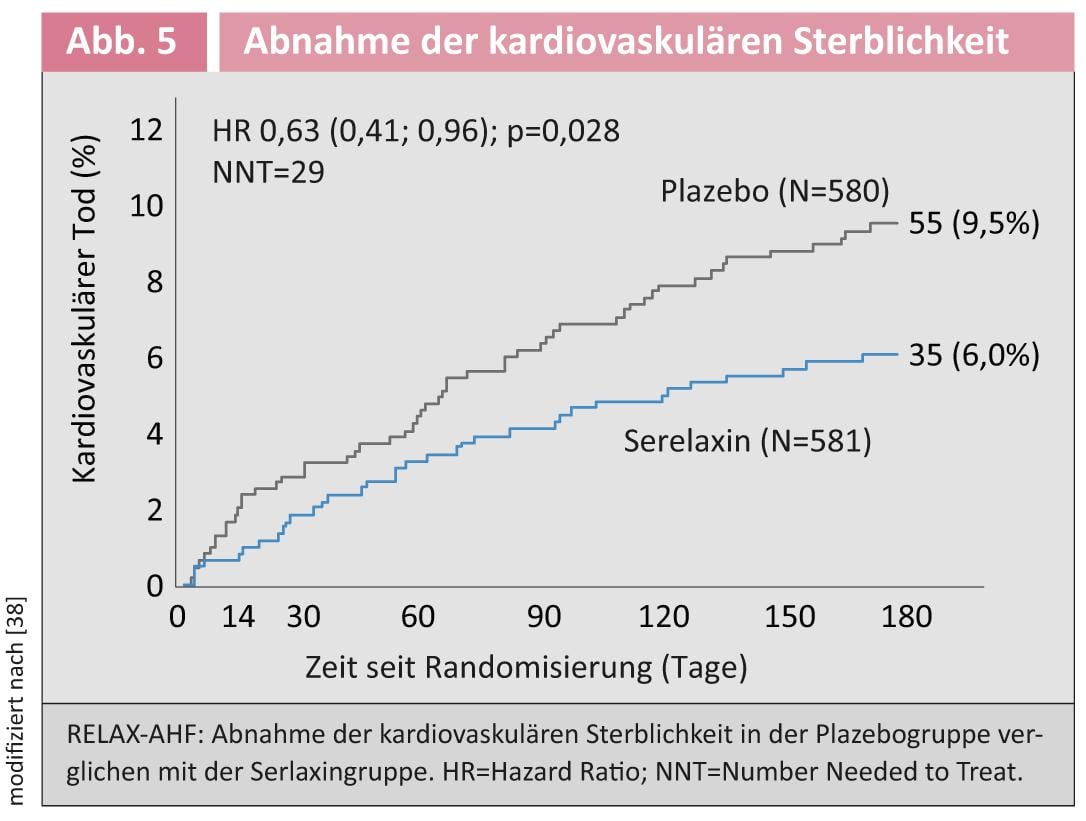
Although this was not a defined endpoint, it is consistent with the results of the Pre-RELAX-AHF study [24]. In this respect, this could be a positive approach for patients with acute heart failure. However, another controlled trial with mortality as the primary end point needs to be conducted to definitively address this question.
Ultrafiltration: patients with heart failure often suffer diuretic resistance due to the need to administer high-dose diuretics, which has a poor prognosis [25]. In the course of recompensation, complicating further deterioration of renal function up to acute renal failure often occurs. A recently published study investigated whether this adverse effect of intravenous diuretics can be remedied by ultrafiltration. Here, ultrafiltration was studied versus optimal pharmacological therapy including a diuretic [17]. Contrary to the hypothesis, renal function tended to deteriorate more in the ultrafiltration group, although water elimination was comparable in both groups. Secondary end points (body weight, NT-pro-BNP concentration, mortality) were not significantly different. There was a more frequent occurrence of side effects in the group of ultrafiltrated patients. In summary, these data indicate that the role of ultrafiltration in acute heart failure and impaired renal function is not superior to standard therapy.
Janine Pöss, MD
Conflicts of interest: MB received speaker fees from Servier, Boehringer Ingelheim, Medtronic, St. Jude Medical, and Bayer.
Literature:
- McMurray JJ, et al. (Guidelines ESCCfP): ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787-1847.
- Böhm M (2013) CardioUpDate Handbook Cardiology ISBN 978-3-86302-065-1
- McMurray JJ, et al: Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362: 767-771.
- Pitt B, et al:The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709-717.
- Zannad F, et al. (Group E-HS): Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11-21.
- Chatterjee S, et al: Eplerenone is not superior to older and less expensive aldosterone antagonists. Am J Med 2012; 125: 817-825.
- Reil JC, et al: Heart rate reduction in cardiovascular disease and therapy. Clin Res Cardiol 2011; 100: 11-19.
- Swedberg K, et al: Rationale and design of a randomized, double-blind, placebo-controlled outcome trial of ivabradine in chronic heart failure: the Systolic Heart Failure Treatment with the I(f) Inhibitor Ivabradine Trial (SHIFT). Eur J Heart Fail 2010; 12: 75-81.
- Swedberg K, et al: Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376: 875-885.
- Borer JS, et al: Effect of ivabradine on recurrent hospitalization for worsening heart failure in patients with chronic systolic heart failure: the SHIFT Study. Eur Heart J 2012; 33: 2813-2820.
- Swedberg K, et al: Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose?: findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol 2012; 59: 1938-1945.
- Komajda M, et al: Influence of background treatment with mineralocorticoid receptor antagonists on ivabradine’s effects in patients with chronic heart failure. Eur J Heart Fail 2013; 15: 79-84.
- Dickstein K, et al. (Committee for Practice Guidelines of the European Society of C): 2010 focused update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur J Heart Fail 2010; 12: 1143-1153.
- Tang AS, et al. (Resynchronization-Defibrillation for Ambulatory Heart Failure Trial I): Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010; 363: 2385-2395.
- Healey JS, et al: Cardiac resynchronization therapy in patients with permanent atrial fibrillation: results from the Resynchronization for Ambulatory Heart Failure Trial (RAFT). Circ Heart Fail 2012; 5: 566-570.
- Mebazaa A, et al: Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 2007; 297: 1883-1891.
- Bart BA, et al. (Heart Failure Clinical Research N): Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367: 2296-2304.
- Felker GM, et al: Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol 2003; 41: 997-1003.
- Massie BM, et al: Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010; 363: 1419-1428.
- O’Connor CM, et al: Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011; 365: 32-43.
- Konstam MA, et al. (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan I): Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007; 297: 1319-1331.
- Conrad KP: Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol 2011; 301: R267-275.
- Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M, Investigators REiAHF (2013) Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 381:29-39
- Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, Marmor A, Katz A, Grzybowski J, Unemori E, Teichman SL, Cotter G (2009) Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 373:1429-1439
- Felker GM, et al. (Network NHFCR): Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364: 797-805.
The further bibliography can be requested from the author.