The Global Initiative for Asthma (GINA) has also updated its recommendations for asthma management in 2020. The most important announcement in it for the occasion: during the COVID-19 pandemic, patients should absolutely continue their prescribed therapies. And something has also happened in the treatment of severe asthma.
Those who abruptly discontinue their medications on their own authority due to SARS-CoV-2 risk worsening their asthma control, which in the worst-case scenario can lead to severe exacerbations. However, the fear of many patients is unfounded anyway, the GINA authors write, because neither inhalable corticosteroids nor biologics or short-term administration of oral corticosteroids in combination with COVID-19 pose a risk.
With regard to mild asthma, the recommendations were made more specific: For stage 1 patients with only occasional symptoms, the fixed combination of an inhaled corticosteroid (ICS) and the betamimetic formoterol is recommended as needed. Alternatively, continuous therapy can be managed with an ICS and a beta-mimetic administered via a second inhaler if needed. In stage 2, ICS/formoterol therapy is now equivalent to continuous therapy consisting of an inhaled corticosteroid and a betamimetic as needed. In addition, the information on the start of therapy was made more specific: Patients who do not experience symptoms on a daily basis should enter stage 2, patients who experience symptoms more frequently but rarely wake up at night should enter stage 3, and all others should enter stage 4.
Dupilumab option for severe asthma
Not revolutionary news, but now also included in the GINA recommendations: The monoclonal interleukin (IL)-4 antibody dupilumab is included in stage 5 as a new add-on therapy option for the treatment of severe asthma (tab. 1). The biologic is approved in Switzerland for the treatment of bronchial asthma from the age of 12.
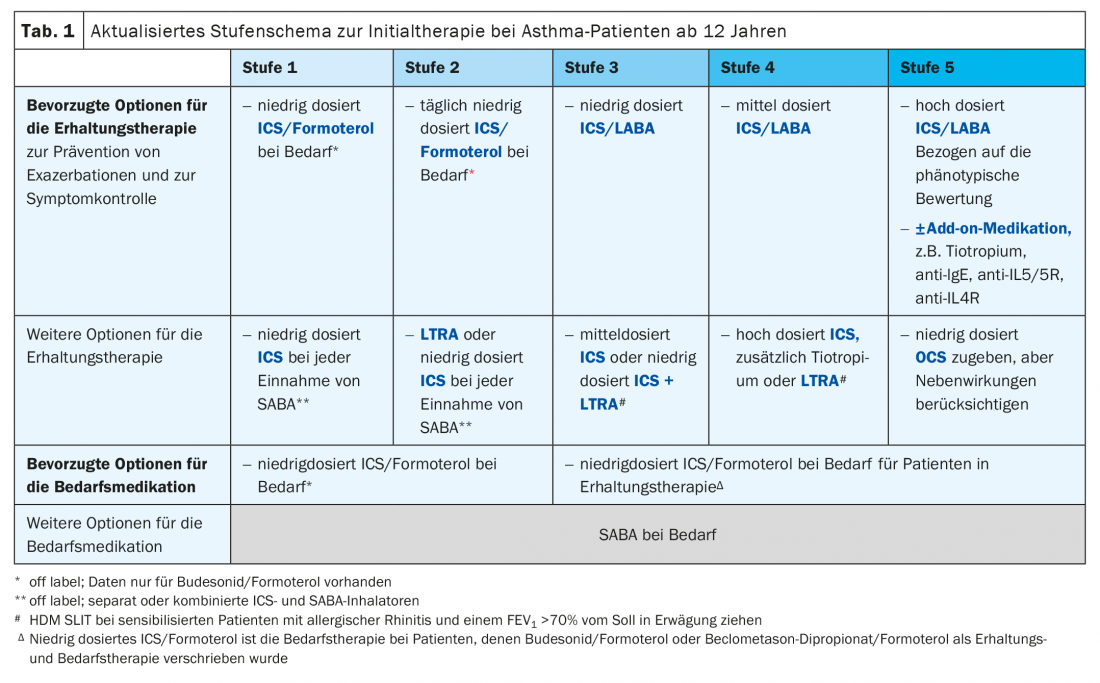
The GINA recommendation is for adults and adolescents 12 years of age and older with severe eosinophilic/type 2 asthma at a dose of 200 mg or 300 mg injected s.c. every two weeks and at a dose of 300 mg s.c. every two weeks for patients requiring OCS. In addition, dupilumab may be considered for sufferers who have concomitant moderate or severe atopic dermatitis.
Literature:
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2020; ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf.
InFo PNEUMOLOGY & ALLERGOLOGY 2021; 3(1): 26.