Anticoagulation must be individualized to the support system and patient. Patients with non-pulsatile systems have been shown to have acquired von Willebrand syndrome, which increases the risk for bleeding complications. Studies with new oral anticoagulants have not yet been performed in patients with cardiac assist devices. Therefore, there is no approval of new oral anticoagulants for LVAD patients.
Ventricular assist devices (VADs) are now an established therapy for terminal heart failure. For example, more left ventricular assist devices than hearts were implanted worldwide in 2012. In Switzerland, 33 heart transplants performed last year were compared to 116 patients on the waiting list for heart transplantation. The waiting time for a donor heart was 179 days in 2009. This explains the increasing importance of support systems that are available without limits.
Although long-term data of more than ten years are not yet available, the 1- and 2-year survival rates after VAD implantation are very promising, and many systems are now used not only as a bridge to heart transplantation but also as a definitive solution.
VAD systems in Switzerland
In cardiac support systems, one must distinguish between pulsatile and non-pulsatile systems. Nonpulsatile pumps have become predominant in left ventricular assist devices. Pulsatile pumps are primarily used for long-term biventricular or right ventricular cardiac support.
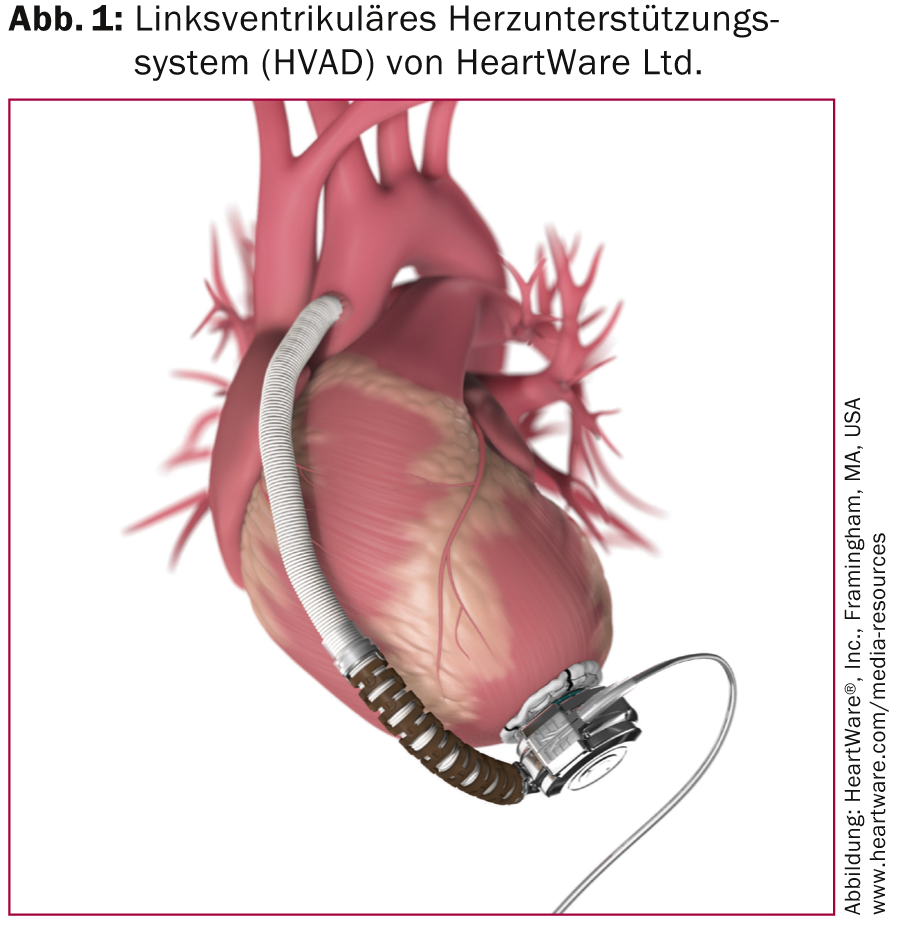
The most commonly implanted left ventricular assist devices are the HVAD (Figure 1) and the HeartMate II (Figure 2).

A partial overview of the systems on the market is shown in Table 1 .

Anticoagulation
Post-operatively, however, anticoagulation should also be adjusted individually to the patient, depending on the pump.
Temporary support systems: Temporary systems are usually continuously anticoagulated with heparin for the short duration. According to company data, an ACT of >300 s should be present at implantation. Later in the course, the target ACT is between 160 and 180 s or the target PTT is a 1.5-1.8-fold increase [1, 2].
Non-pulsatile long-term support systems: In most cases, implantation of a VAD is performed using the heart-lung machine; therefore, administration of heparin with an ACT >400 s intraoperatively is required. If implantation is performed without a heart-lung machine, an ACT of >200 s should be achieved during implantation. After termination of the heart-lung machine or at the end of the operation, heparin is completely antagonized.
Further anticoagulation should not be started until after 24 h. Target PTT ranges from 40 to 60 s on the first to second postoperative day.
For centrifugal pumps, the target PTT should be increased to 60-80 s from the second to third post-operative day. After switching to warfarin, the recommended INR is between 2.0 and 3.0. Additional platelet aggregation, e.g. aspirin (81-325 mg/tgl), can be started from the first post-operative day and it should be administered at the latest after the chest drains are pulled.
For the HeartMate II axial pump, the target PTT is slightly lower (60-75 s) from the second to third post-operative day, and the target INR after switching to warfarin is between 2.0 and 3.0 (according to company data). As platelet aggregation, aspirin is 81-325 mg/tgl. recommended [3].
After individual dose adjustment of warfarin, platelet aggregation should additionally be checked by platelet aggregation test or, if clopidogrel is administered, by VASP test and adjusted individually.
Pulsatile long-term support systems: For the Thoratec PVAD, heparin, target PTT with 1.5-fold increase, should be started after reduction of bleeding to less than 50 ml/h. When switching to warfarin, the target INR is 2.5-3.5. As platelet aggregation, 100 mg aspirin can be added daily [4].
Excor requires an INR of 3.0-3.5 in adults and platelet aggregation of at least 75 mg per day of aspirin and dipyridamole at a minimum of 150 mg per day, which should be adjusted for platelet counts [5].
For outpatient follow-up, each patient should be provided with a Coagu-Check.
Coagulation disorders: Acquired von Willebrand syndrome has been demonstrated in some non-pulsatile systems. Shear forces in axial and centrifugal pumps alter the structure of von Willebrand factor, allowing it to be cleaved by the metalloproteinase ADAMTS13 [6]. Therefore, there is an absence of the large multimers that are primarily responsible for primary hemostasis (Fig. 3).

Furthermore, the extent to which platelet alteration occurs as a result of the assist pumps is not entirely clear. Thus, damage to platelet receptors (GPIb-V-IX) by shear stress [7], but also decreased platelet aggregation by cleavage products of von Willebrand factor are discussed [8, 9].
Studies with new oral anticoagulants have not yet been performed in patients with cardiac assist devices. A trial of low-molecular-weight heparin until discontinuation with warfarin after implantation of a left ventricular assist device [10] showed an increased rate of stroke in the first 30 days after deduction of the perioperative time of 48 h with 2.5 vs. ≤0.7% [11, 12].
Anticoagulation for complications
Bleeding: multivariable risk factors for increased bleeding rate after VAD implantation include age, female sex, ischemic cardiomyopathy, and decreased preoperative hematocrit [13]. The extent to which matched anticoagulation provides improvement for these subgroups remains to be investigated.
In patients with gastrointestinal bleeding, an algorithm by Suarez et al. [14, 15], according to which complete anticoagulation should first be paused. If the source of bleeding is identified and treated, warfarin should be started first and platelet aggregation inhibition should continue to be paused. If bleeding complications continue, the INR can be reduced to 1.5-2.0.
To stop bleeding when the source of bleeding is not identified, administration of clotting factors such as prothrombin concentrates or Hemate®, platelet concentrates, octreotide, and desmopressin is recommended.
Pump thrombosis: In patients with suspected pump thrombosis with elevated hemolysis parameters, coagulation should be optimized first and continuous heparin should be given if necessary. If suspicion persists, the administration of thrombin inhibitors is recommended. If this does not result in successful treatment, consideration must be given to replacing the ventricular assist device or performing lysis therapy [16]. If cured, an elevated INR with the addition of a second antiplatelet agent should be considered in the further course [17].
Anticoagulation during surgery: According to guidelines from the American College of Chest Physicians, warfarin should be discontinued five to six days before and an antiplatelet agent should be discontinued seven days before and switched to heparin before a planned surgery with an increased risk of bleeding [18]. Guidelines for VAD patients do not exist for noncardiac surgery; however, in an article by Morgan et al. be shown that switching from warfarin and ASA to heparin with perioperative pausing of heparin resulted in fewer bleeding complications [19]. FFPs, prothombin concentrates, or vitamin K may be administered during emergency or urgent surgery [3]. For minor surgeries such as tooth extractions or skin surgery, continued anticoagulation with warfarin and ASA is recommended in non-LVAD patients. Mouth rinsing with tranexamic acid and local hemostasis with collagen sponges and fibrin glue as well as the use of a dental splint can additionally prevent bleeding [20].
Anna L. Meyer, MD
Literature:
- Oliver WC: Anticoagulation and coagulation management for EMCO. Semin Cardiothorac Vasc Anesth 2009; 13(3): 154-175.
- http://columbialvad.org/pdf/centrimag.pdf.
- Feldmann D, et al: The 2013 International Society for Heartand Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013; 32(2): 157-187.
- www.thoratec.com/_assets/download-tracker/15579-A.pdf.
- www.berlinheart.de/UserFiles/Downloaddokumente/Medical_Professionals_Distributoren/Non_US/EXCOR_Parakorporales_Herzunterstuetzungssystem/Gebrauchsanweisungen/EXCOR_VAD_Ikus_Antrieb/Ikus_Rev_2_1_Software_3_40/Gebrau3.40AMRev80en.pdf.
- Tsai HM: Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood 1996; 87(10): 4235-4244.
- Himmelfarb J, et al: Elevated plasmaglycocalicin levels and decreased ristocetin-induced platelet agglutination in hemodialysis patients. Am J Kidney Dis 1998; 32(1): 132-138.
- Sugimoto M, et al.: Functional modulation of the isolated glycoprotein Ib binding domain of von Willebrand factor expressed in Escherichia coli. Biochemistry 1991; 30(21): 5202-5209.
- Alevriadou BR, et al: Real-time analysis of shear-dependent thrombus formation and its blockade by inhibitors of von Willebrand factor binding to platelets. Blood 1993; 81(5): 1263-1276.
- Sandner SE, et al: Low-molecular-weight heparin for anti-coagulation after left ventricular assist device implantation. J Heart Lung Tranplant 2014; 33(1): 88-93.
- Starling RC, et al: Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2011; 57(19): 1890-1898.
- Miller LW, et al: Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007; 357(9): 885-896.
- Boyle AJ, et al: Pre-Operative Risk Factors of Bleeding and Stroke During Left Ventricular Assist Device Support: An Analysis of More Than 900 HeartMate II Outpatients . J Am Coll Cardiol 2014; 63(9): 880-888.
- Suarez J, et al: Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Heart Fail 2011; 4(6): 779-784.
- Tamez D, et al: Early Feasibility Testing and Engineering Development of the Transapical Approach for the HeartWare MVAD Ventricular Assist System. ASAIO J 2014; 60(2): 170-177.
- Schlendorf K, et al: Thrombolytic therapy for thrombosis of continuous flow ventricular assist devices. J Card Fail 2014; 20(2): 92-97.
- Goldstein DJ, et al: Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 2013; 32(7): 667-670.
- Douketis JD, et al: Perioperative management of antithombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis,9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 Suppl): e326S-350S.
- Morgan JA, et al: Non-cardiac surgery in patients on long-term left ventricular assist device support. J Heart Lung Transplant 2012; 31(7): 757-763.
- Lund JP, et al: Oral surgical management of patients with mechanical circulatory support. Int J Oral Maxillofac Surg 2002; 31(6): 629-633.
CARDIOVASC 2014; 13(2): 18-20