New data on first-line treatment of metastatic colorectal cancer were presented at the ASCO GI in San Francisco. The post-hoc analyses consistently confirm that RAS mutation status is an important component in treatment decisions and should definitely be clarified.
New results on subanalyses of the mutational status of metastatic colorectal cancer (mCRC) tumors from the FIRE-3, the OPUS study and also the CECOG/CORE2 study bring medicine another step closer to personalized treatment of mCRC.
Survival benefit with cetuximab in wild-type RAS.
The FIRE-3 trial was the first Phase III head-to-head study testing the antibodies cetuximab and bevacizumab against each other. The patient population is patients with KRAS wt mCRC who received first-line treatment. The primary endpoint of the study is overall response rate. A total of 592 patients were treated in 150 centers between 2007 and 2012. Randomization was 1:1 into two treatment arms: one was chemotherapy FOLFIRI plus cetuximab, the other was FOLFIRI in combination with bevacizumab. Although the primary endpoint of the study was not met, with overall response rates differing by only 4% between the two treatment groups, there was an overall survival benefit of 3.7 months (HR=0.77; p-value=0.017) in the Erbitux® (cetuximab, n=297) treatment group.
In his presentation at the ASCO GI in San Francisco in January 2014, PD Sebastian Stintzing, MD, Munich, provided information on the evaluation of the different subgroups. In this analysis, the effect of different tumor mutations in the EGFR signaling chain was investigated. The patient population was analyzed for KRAS (exon 2,3, and 4), NRAS (exon 2,3, and 4), BRAF, and PIK3CA mutations, and the outcomes of each of the two treatment arms on overall response rate, progression-free survival, and overall survival were considered.
Also, as in the overall analysis, splitting the results by mutation type showed that the treatment arms did not differ significantly in overall response rate and progression-free survival, and patients benefited comparably from the treatments. “But if we look at the overall survival results in all RAS wild types, we see a clear advantage of the combination therapy of chemotherapy FOLFIRI and cetuximab,” PD Dr. Stintzig emphasized. Comparing the two treatment arms, patients in the cetuximab group benefit from an additional 7.5 months of overall survival with a hazard ratio of 0.7 (Fig. 1).
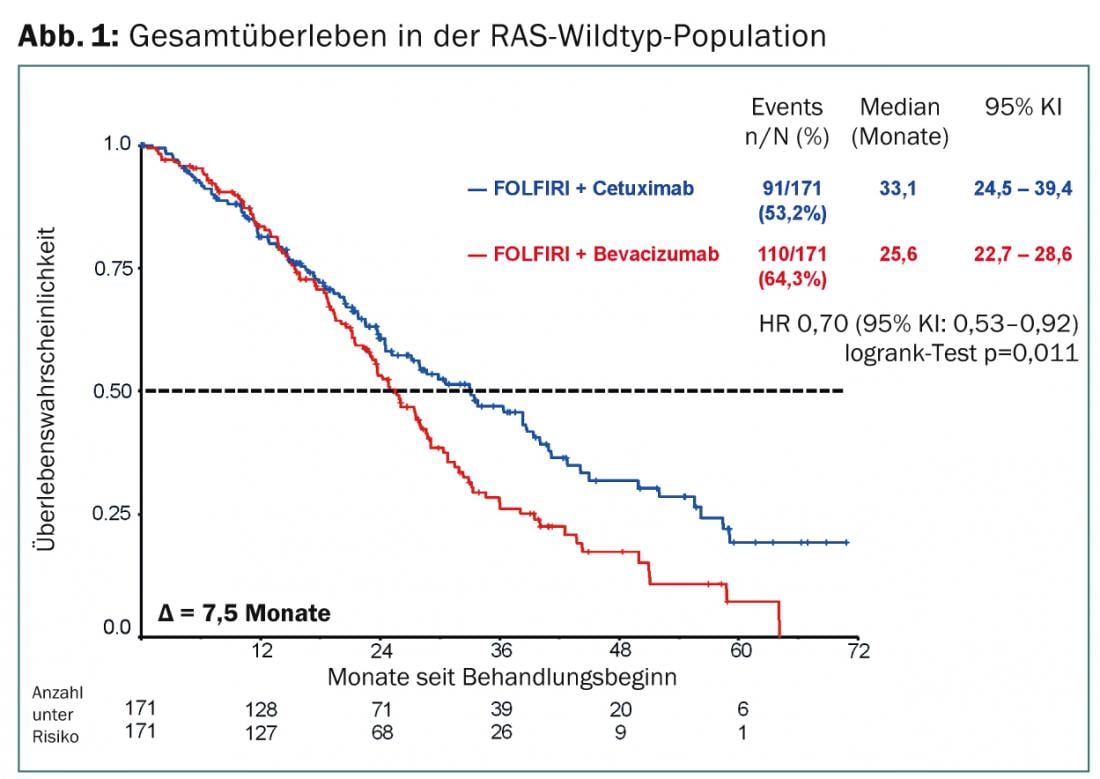
The difference in median survival of 33.1 vs. 25.6 months is statistically significant with a p-value of 0.011. Why patients clearly benefit with regard to overall survival under cetuximab but this does not apply to progression-free survival is still unclear, and further research is needed here.
“For patients who have RAS-mutated tumors, no superiority was shown for any of the treatment arms,” PD Dr. Stintzing added. As shown in previous analyses, this again confirms that RAS mutation status is a major key to treatment fit in mCRC.
Looking at the patient subgroup with BRAF-mutated tumors, comparable results were seen in terms of overall response rate, progression-free survival and overall survival. The situation is similar for the treatment of PIK3CA mutations: There were no statistically significant differences in the two combination therapies in terms of overall response rate and survival. Progression-free survival was longer for PIK3CA mutations in the treatment arm with bevacizumab, but this result was also not statistically significant.
The results of the new subgroup analysis can be summarized as follows:
- Clarification of RAS(KRAS and NRAS) mutation status is critical for adequate therapy selection in mCRC patients prior to treatment.
- Patients of all RAS wild-type had a clinically relevant survival benefit when first-line treatment consisted of a combination of cetuximab plus chemotherapy FOLFIRI.
- The effect on overall survival was comparable for both cetuximab and bevacizumab in patients with BRAF- or PIK3CA-mutated tumors.
News from OPUS and CECOG/CORE2 study
New results from the OPUS study were also presented in San Francisco. The OPUS trial is a Phase II study that evaluated the efficacy of cetuximab in combination with FOLFOX4 chemotherapy compared to monotherapy with FOLFOX4. It was shown that mCRC patients with RAS wild-type tumor status benefit significantly from first-line cetuximab therapy [1]. This applies to both response rate and progression-free survival. The response rate of combination therapy with cetuximab was 61.1%, significantly higher than the rate of chemotherapy alone at 30.4%, and the difference was statistically significant. The progression-free survival outcome was also significantly better in favor of combination therapy at 12 vs. 5.8 months (HR=0.43; p=0.018). In contrast, patients who have a KRAS or NRAS mutation do not benefit from the addition of cetuximab to FOLFOX4. To maximize patient benefit, the authors recommend administering the combination therapy to patients with wild-type tumors, but not if they have a mutation. However, due to the small number of patients, further studies are needed to validate the results.
Analyses of mutational status from the CECOG/CORE2 trial also confirm its importance in first-line treatment in mCRC: RAS wild-type patients treated with combined therapy of FOLFOX4 and cetuximab (either weekly or every two weeks) had a significant prolongation of overall survival of 28.5 vs. 16.3 months in RAS mutated patients (HR=0.43; p=0.0199) [2]. However, the results regarding overall response rate and progression-free survival did not reach statistical significance.
Source: “Oral Abstract Session: Cancers of the Colon and Rectum” ASCO GI – Gastrointestinal Cancers Symposium, January 16-18, 2014, San Francisco.
Literature:
- Tejpar S, et al: Effect of KRAS and NRAS mutations on treatment outcomes in patients with metastatic colorectal cancer (mCRC) treated first-line with cetuximab plus FOLFOX4: New results from the OPUS-study. Poster LBA444, presented at ASCO GI 2014, San Francisco.
- Brodowicz T, et al: FOLFOX4 plus cetuximab administered weekly or every two weeks in first-line treatment of patients with KRAS and NRAS wild-type (wt) metastatic colorectal cancer (mCRC). Poster LBA391, presented at ASCO GI 2014, San Francisco.
InFo Oncology & Hematology 2014; (2)1: 21-22.











