The treatment spectrum of inflammatory dermatoses has greatly expanded. The 2019 re-approval of risankizumab (psoriasis) and dupilumab (atopic dermatitis) are just some of the milestones. It can be assumed that further new drugs will soon be launched on the market.
Research into the pathogenetic function of immunomodulators forms an important basis for the development of new target-specific agents. PD Dr. med. Robert Sabat, Head of the Psoriasis Research and Treatment Center, Charité-Universitätsmedizin Berlin, gave an up-to-date overview of immunological principles with a focus on the most common inflammatory dermatoses at this year’s Zurich Dermatological Training Days [1].
Psoriasis as a prime example
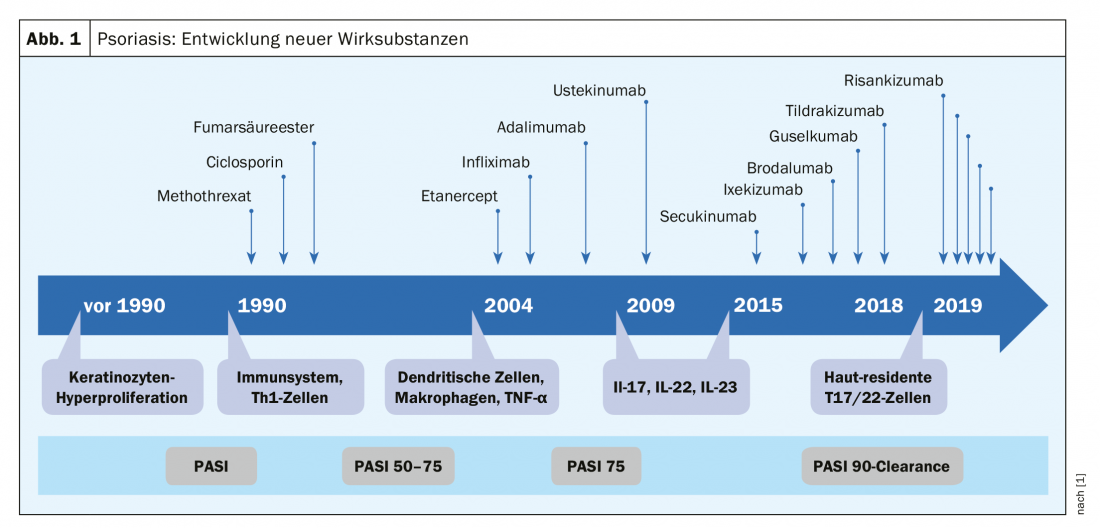
The neutralization of individual cytokines – pathogenetic key players of inflammatory skin diseases and targets of specific therapies – revolutionized the treatment options of several dermatoses. Psoriasis is a good example of this: Over the past two decades, numerous new pharmacotherapeutic agents have made breakthroughs, with the greatest advances occurring in the past few years (Fig. 1). In 2018 alone, several new agents have entered the market (ixekizumab, brodalumab, guselkumab, tildrakizumab). “Psoriasis is a prime example that understanding inflammatory processes really pays off,” the speaker explained. According to Dr. Sabat, the pathomechanism of psoriasis can be summarized as an excessive tissue reaction to certain cytokines of certain immune cells. Currently, the focus is on skin-resident Th17 and Th22 cells and the therapeutic target is PASI90 or PASI100. With regard to atopic dermatitis, the new approval in 2019 of the monoclonal antibody dupilumab, as the first biologics representative for this indication, represents a milestone [6].
The fact that atopic dermatitis and psoriasis are common skin diseases is also shown by new epidemiological figures. Regarding psoriasis, the Danish Skin Cohort prospective study published in 2019 reported a lifetime prevalence of 7.9% and a 1-year prevalence of 5% in a representative sample of the general population (n=3490) [2]. In contrast to atopic dermatitis and psoriasis, much less is known about the exact phenotypes of disease-relevant T cells in many other immune-mediated skin diseases (e.g., lichen planus, acne inversa/hidradenitis supurativa, or pemphigus) [3]. But intensive research efforts are underway in this regard as well. For example, a recent study on cytokine profiles of psoriasis and acne inversa shows that cutaneous IL-1β levels are significantly higher in acne inversa/hidradenitis supurativa than in psoriasis [4].
Cytokines as key players
T-cell mediated skin diseases such as psoriasis, atopic dermatitis, vitiligo and acne inversa follow the identical scheme: cell sensitization phase, effector phase, clinical symptoms. In the cell sensitization phase, interaction between dendritic cell and T cell occurs. When some of these T cell populations are overactivated, chronic inflammatory diseases result. In psoriasis this affects the T cell populations Th17 and Th22, in vitiligo Th1, in atopic dermatitis mainly Th2 cells. Pathogenetically relevant in psoriasis are the Th17-induced secretion of the cytokines IL-17, IL26, IL-29 resp. Th22-induced secretion of the cytokines IL22 and TNFα. Inhibition of these key cytokines underlies the new biologicals/biosimilars developed in recent years [1,6]:
TNFα inhibitor: etanercerpt, infliximab, adalimumab, certolizumab pegol, golimumab. Except for golimumab (psoriatic arthritis only), all of the above agents are approved for psoriasis and psoriatic arthritis.
IL17 inhibitor: secukinumab, ixekizumab. Both are also approved for psoriatic arthritis.
IL23 inhibitor: ustekinumab , guselkumab, tildrakizumab, risankizumab. Ustekinumab is approved for psoriasis and psoriatic arthritis, the others only for psoriasis.
Risankizumab (SKYRIZI™), approved in 2019 [5], can be used to treat moderate-to-severe plaque psoriasis in adult patients who have had an inadequate response to other systemic therapies, such as cyclosporine, methotrexate (MTX), or PUVA (psoralen and UV-A), or who have a contraindication or intolerance to such therapies [5].
Regarding atopic dermatitis, the 2019 marketing authorization of dupilumab (Dupixent®), as the first representative of biologics for this indication, is the latest evolution of treatment options [6].
Dr. Sabat points out that research into pharmacological therapeutics for inflammatory dermatoses is far from complete. Phase II and Phase III trials are currently underway for several compounds and it is expected that the spectrum of therapeutic options will expand again in the near future.
Literature:
- Sabat R: Slide presentation: Update Course 3 – Inflammatory Dermatoses. Pathophysiology of inflammation of the skin: overview lecture, PD Robert Sabat, MD, 9th Zurich Dermatology Training Days, Zurich, June 27, 2019.
- Egeberg A, Andersen YMF, Thyssen JP: Prevalence and characteristics of psoriasis in Denmark: findings from the Danish skin cohort BMJ Open 2019; 9:e028116. doi: 10.1136/bmjopen-2018-028116.
- Sabat R, et al: T cell pathology in skin inflammation. Seminars in Immunopathology 2019; 41 (3), 359-377.
- Witte-Händel E, et al: The IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J Invest Dermatol 2019; 139(6): 1294-1305. doi: 10.1016/j.jid.2018.11.018. epub 2018 Dec 5.
- Swiss Drug Compendium: SKYRIZI™. https://compendium.ch/, last accessed June 28, 2019.
- Swiss Drug Compendium: Dupixent®. https://compendium.ch/, last accessed June 28, 2019.
DERMATOLOGIE PRAXIS 2019; 29(4): 32-33 (published 8/26/19, ahead of print).