Lymphoma accounts for 3.8% of all new cancer diagnoses. According to the current WHO classification, there are over 75 different lymphomentities, which are treated differently. In the treatment of diffuse large B-cell lymphoma (DLBCL), peripheral T-cell lymphoma and classical Hodgkin lymphoma, a lot has happened over the last years.
According to previously used classifications, lymphomas are traditionally divided into so-called Hodgkin and non-Hodgkin lymphomas. This classification is still used in individual statistics and serves as the basis for national incidence recording (www.nkrs.ch/de/stat). According to this data collection, nearly 1600 patients per year in Switzerland develop non-Hodgkin’s lymphoma and approximately 250 develop Hodgkin’s lymphoma. Lymphoma accounts for 3.8% of all new cancer diagnoses. Annual mortality is just over 500 deaths. Thus, lymphoma accounts for 3.2% of all cancer deaths.
Current WHO classification and clinical presentation
The World Health Organization (WHO) has already established several classifications for lymphomas and has increasingly replaced the term non-Hodgkin’s lymphoma with clear naming and subtyping of individual entities. The currently valid classification from 2016 includes more than 75 lymphomentities [1]. It is based on the different morphology, clinical presentation and increasingly also on molecular, respectively genetic aspects.
This classification is very important from my point of view, because diagnosis, prognosis as well as therapy differ fundamentally in some cases. The terms indolent and aggressive should nowadays be used primarily in reference to the growth behavior of the respective lymphoma. In this context, approximately 50% of all lymphomas belong to the group of slow-growing lymphomas and are thus referred to as indolent. The other 50% are fast-growing and therefore rather aggressive lymphomas. In this compilation, I would like to briefly discuss the therapy of selected aggressive lymphomas.
Therapy of diffuse large B-cell lymphoma (DLBCL)
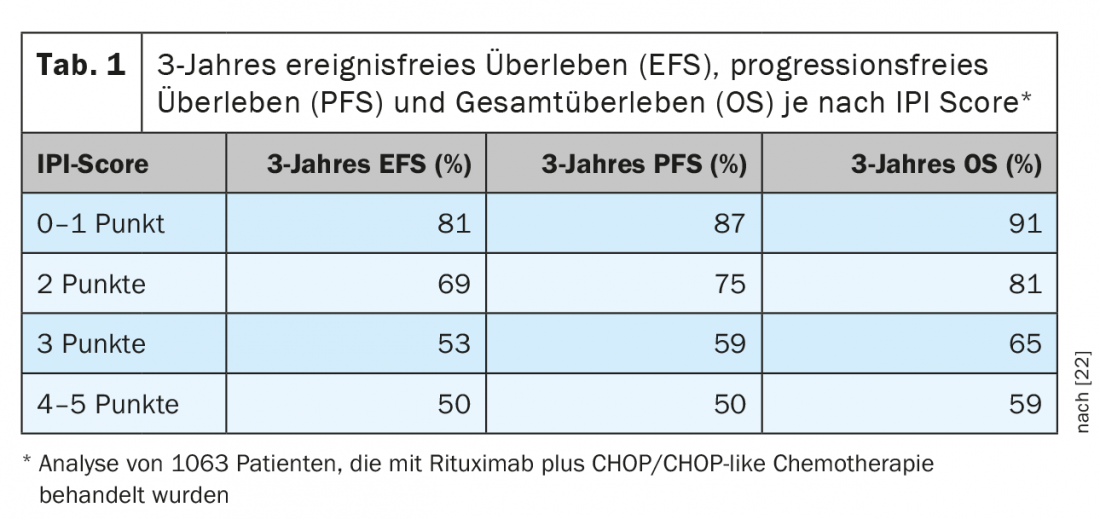
DLBCL account for 25-30% of all lymphomas, making it the most common lymphoma entity. They belong to the aggressive B-cell lymphomas. The goal of treatment in most cases should be long-term disease control or cure. This is usually achieved only by means of intensive immunochemotherapy, which is based on the combination of a CD20-specific antibody with poly-chemotherapy. Typically, a CHOP or CHOP-like regimen is used. With regard to forecasting, various evaluation systems now exist. The longest used and mostly still valid risk stratification is based on the so-called “International Prognostic Index (IPI)” (Table 1) [2]. For patients under 60 years of age, the adapted, so-called “age-adjusted International Prognostic Index (aaIPI)” is often used. Based on this classification, DLBCL patients can be divided into four groups. While over 60-year-olds are not further subtyped, there are three groups in the under 60-year-olds: Early stage with favorable prognosis (aaIPI 0), younger patients with unfavorable prognosis (aaIPI 1 and/or bulk) and as a third group so-called high-risk patients (aaIPI ≥2).
Six cycles of rituximab + CHOP are often used to treat elderly patients (>60 years), primarily at 21-day intervals and rarely every 14 days. The superiority of a 14-day regimen noted on the part of the German DSHNL study group [3] could not be confirmed by either the English [4] or the French study groups [5], although there is no direct comparability between the studies. The benefit of eight cycles of R-CHOP compared with six cycles in the 21-day regimen was not randomly tested. However, retrospective analyses of the randomized phase III GOYA trial [6], in which centers were given the choice of six or eight cycles, showed no difference in efficacy. Thus, six cycles of R-CHOP every 21 days is often considered standard. Also, twice additional administration of immunotherapy as seventh and eighth treatment cycles in the form of two further rituximab administrations in the so-called “6 + 2 regimen” did not prove to be superior in the PETAL study [7].
In younger patients under 60 years of age with absence of risk factors and favorable prognosis (aaIPI 0, no bulk), four cycles of R-CHOP-21 was shown to provide excellent progression-free survival (PFS) and overall survival (OS) in the recently published FLYER trial [8]. This allows for dose reduction and significant reduction in treatment-related morbidity with high cure rates (3-year PFS 96% vs. 93%). For the second group of under 60-year-olds with aaIPI 1 and/or bulk, six cycles of R-CHOP-21 with consolidative radiotherapy of the initial bulk is considered standard. In the MINT study of DSHNHL [9], the 6-year event-free survival (EFS) with this therapy was 71%. The French GELA/LYSA study group showed superiority of the R-ACVBP regimen [10] over R-CHOP-21 for this collective. However, this result must be critically questioned because, in contrast to the DSHNHL study, no radiotherapy was used.
Younger high-risk patients (aaIPI ≥ 2) unfortunately continue to have a higher risk of recurrence and thus lower chances of cure. The optimal therapy for this patient population is not uniformly defined. Some study groups use the R-CHOP-21 scheme mentioned earlier [4]. Other study centers, such as individual U.S. and European centers, perform a priori consolidative high-dose therapy with autologous stem cell transplantation in the first line of therapy. DSHNHL tested eight R-CHOEP-14 cycles against a triple-transplant approach in the MEGA-CHOEP trial [11]. Superiority (3-year EFS 69.5% vs 61.4%) of the 8 x R-CHOEP-14 arm was demonstrated. In the U.S. scope, the R-DA-EPOCH is often used instead of the R-CHOEP regime. In general, most centers favor a more dose-intensive treatment regimen than a standard R-CHOP regimen.
In particular, the therapy of younger high-risk patients shows that first-line therapy of DLBCL is crucial for prognosis. If disease relapse occurs within 12-18 months, the prognosis is often infaust and only a few patients can still achieve long-term remission. However, if patients have not had a recurrence in the first 18 -24 months after the end of therapy, long-term disease control with high cure rates is much more common. This indicates that a curative approach, if possible, should be adopted in first-line therapy. In recent years, molecular biology has allowed us to gain a deeper insight into the underlying pathomechanisms of the disease and various studies, based on the already mentioned and established R-CHOP regimen, have studied the therapeutic value of different signaling inhibitors. For example, the proteasome inhibitor bortezomib has also been tested in Switzerland [12]. Unfortunately, no treatment benefit was seen in the overall population. The addition of the immunomodulator lenalidomide to R-CHOP also showed no significant benefit in the prospectively randomized ROBUST trial [13]. Studies are currently underway on the use of BTK inhibitors (e.g., ibrutinib) [14], as well as BCL2 inhibitors (venetoclax) [15]. Initial phase II studies and subgroup analyses, respectively, suggested certain benefits for the latter two compounds. However, it has not yet been proven that these agents show significant superiority over the current standard and thus neither BTK, nor BCL2 inhibitors are currently the subject of our daily routine. As a new therapeutic concept, in the case of recurrence, i.e. in older patients after two previous therapies and in younger patients usually after two previous therapies incl. High-dose therapy with autologous stem cell replacement, establishing CAR T-cell technology [16]. This is available in Switzerland at specialized centers.
Therapy of aggressive peripheral T-cell lymphomas (PTCL)
T-cell lymphomas account for approximately 10% of all new lymphoma cases in the Western world. The histopathologic and molecular classification of the individual T-cell lymphoma entities is complex and, unfortunately, due to the rarity of the disease, data from larger randomized trials are often very limited. The prognosis of aggressive T-cell lymphomas is usually less favorable than that of aggressive B-cell lymphomas. Only the so-called “ALK+ peripheral T-cell lymphoma (PTCL)” is often considered to have a more favorable prognosis. The other PTCL subtypes mostly show a rapid disease progression with often only a short-lasting response to therapy. Significant for PTCL treatment is the detection of CD30 antigen on lymphoma cells. If this is expressed, according to the ECHELON-2 trial, a significant improvement in 3-year PFS (57% vs. 44%) and OS can be achieved with the combination of brentuximab vedotin as an immunoconjugate plus CHP chemotherapy [17]. The study compared this treatment with the current standard regimen CHOP. It was left open to the study centers to apply the therapy for six or eight cycles at a time. Brentuximab vedotin, like the vinca alkaloid vincristine, has high neurotoxicity, so in the experimental arm, vincristine had to be stopped when brentuximab vedotin was added. Thus, chemotherapy was reduced from CHOP to CHP. As mentioned above, in addition to the PFS benefit, an OS benefit was also observed. As a consequence, this therapy is currently considered the gold standard for first-line CD30-positive PTCL. Unfortunately, other therapeutic agents such as HDAC inhibitors, immunoconjugates, anti-metabolites and classical chemotherapeutic agents have very limited duration of action in PTCL. Therefore, after the failure of therapy-intensive treatments, often only palliative options with short efficacy are available.
Therapy of classical Hodgkin lymphoma (cHL)
In the treatment of classical Hodgkin lymphoma, many colleagues follow the guidelines of the German Hodgkin Lymphoma Study Group (GHSG). According to GHSG stratification, cHL patients are divided into three risk groups. The last completed and published generation of studies includes the HD16-HD18 studies for therapy of patients with early (HD16), intermediate (HD17) and advanced (HD18) stages. The HD16 trial tested whether consolidative radiotherapy could be omitted in cases of PET negativity after two cycles of ABVD (so-called “PET2 negativity”). According to the results of the study, radiotherapy should not be omitted because the 5-year PFS drops from 93.4% with radiotherapy to 86% without radiotherapy, and thus a significantly worse therapeutic outcome can be expected if radiotherapy is omitted [18].
In the intermediate stage, the HD17 study addressed a similar question [19]. For appropriate patients, standard chemotherapy includes two cycles of BEACOPPPescalated (BEACOPPesc) followed by two cycles of ABVD. After these four therapy cycles, a PET-CT is classically performed (the so-called “PET4”). In the HD17 study, radiotherapy usually administered was omitted in PET4 negativity. Using this treatment regimen showed a 5-year PFS of 97% in the standard arm (with radiotherapy) and of 95.1% in the experimental arm, i.e., omitting radiotherapy in case of PET4 negativity. Thus, treatment with omission of radiotherapy was not inferior to standard therapy when PET findings were appropriate. Thus, according to this study, consolidative radiotherapy can be omitted in PET4-negative patients after four cycles of chemotherapy.
The data from the HD18 study On the treatment of advanced stages of cHL have already been shown at several congresses [20]. Six cycles of BEACOPPesc were defined as the GHSG standard of care in the trial. In the experimental arm, PET2 negativity was tested to determine whether two additional BEACOPPesc cycles – a total of four rather than six – were sufficient. The study demonstrated a 5-year PFS of 91.2% in the standard six-cycle BEACOPPesc arm. The PET2-guided arm showed a comparable result of 91.8%. Thus, a dose reduction to a total of four cycles may now be performed in the case of PET2 negativity. This is a significant advance, particularly given the often young age of cHL patients. Dose reduction can counteract therapy-associated morbidity and, in particular, chemotherapy-induced infertility. Hopefully, with the new standard of care, fewer second neoplasms will occur during life.
CHL is among the entities with the highest response rates to PD1 inhibitor therapy. In the case of recurrence of cHL, I think the publication of the KEYNOTE-204 study is significant for clinical practice [21]. This study tested the use of the PD1 inhibitor pembrolizumab vs. brentuximab vedotin in patients with relapsed cHL in a randomized comparison. In the analysis presented at the 2020 ASCO Annual Meeting, superiority of PD1 blockade was reported with a higher response rate (65% vs. 54%) and longer median PFS of 13.2 vs. 8.3 months. In addition, PD1 blockade showed significantly lower toxicity, especially neurotoxicity, so that in addition to efficacy, tolerability also argues for early use of immunotherapy in the treatment of cHL relapses.
Take-Home Messages
- In the treatment of aggressive B-cell lymphomas, high cure rates can be achieved through the use of immunochemotherapy.
- Therapy of aggressive B-cell lymphomas is risk-stratified with 4-6(8) cycles of immunochemotherapy (R-CHOP or R-CHOP-like).
- Aggressive T-cell lymphomas usually have a much worse prognosis compared to aggressive B-cell lymphomas.
- The use of the CD30 immunotoxin brentuximab vedotin in combination with CHP chemotherapy for CD30-positive PTCL is considered standard.
- cHL is treated with chemotherapy or chemotherapy plus radiotherapy according to stage and risk, and has excellent cure rates. The goal of newer therapeutic approaches is often dose reduction to avoid therapy-related morbidity while maintaining high cure rates.
Literature:
- Swerdlow SH, et al: WHO classification of tumours of haematopoietic and lymphoid tissue. Revised 4th ed. Lyon: IARC; 201.
- The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 1993; 329: 987-994.
- Pfreundschuh M, et al: Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008; 9: 105-116.
- Cunningham D, et al: Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: A phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 2013; 381: 1817-1826.
- Delarue R, et al: Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 2013; 14: 525-533.
- Sehn LH, et al: A randomized, open-label, phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-cell lymphoma: final analysis of GOYA. J Hematol Oncol. 2020; 13(1): 71-79.
- Schmitz C, et al: Impact of complete surgical resection on outcome in aggressive non-Hodgkin lymphoma treated with immunochemotherapy. Cancer Med 2020; 9(22): 8386-8396.
- Poeschel V, et. al: Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, phase 3, non-inferiority trial. Lancet.2019; 394 (10216): 2271-2281.
- Pfreundschuh M, et al: CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011; 12: 1013-1022.
- Recher C et al: Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): An open-label randomised phase 3 trial. Lancet 2011; 378: 1858-1867.
- Schmitz N, et al: Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: An open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol 2012; 13: 1250-1259.
- Davies A, et al: Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. Lancet Oncol. 2019; 20(5): 649-662.
- Lue JK, O’Connor OA.: A perspective on improving the R-CHOP regimen: from Mega-CHOP to ROBUST R-CHOP, the PHOENIX is yet to rise. Lancet Haematol 2020; 7(11): e838-e850.
- Younes A, et al: Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J Clin Oncol 2019; 37(15): 1285-1295.
- Morschhauser F, et al: A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood 2021; 137(5): 600-609.
- Schuster SJ, et al: Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017; 377: 2545-2554.
- Horwitz S, et al: ECHELON-2 Study Group. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 2019; 393(10168): 229-240.
- Fuchs M, et al: Positron emission tomography-guided treatment in early-stage favorable Hodgkin lymphoma: Final results of the international, randomized phase III HD16 trial by the German Hodgkin Study Group. J Clin Oncol 37 2019; 31: 2835-2845.
- Borchmann P, et al: PET-guided omission of radiotherapy in early-stage unfavourable Hodgkin lymphoma (GHSG HD17): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2021; 22(2): 223-234.
- Borchmann P, et al: PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2018; 390(10114): 2790-2802.
- Kuruvilla J, et al: KEYNOTE-204: Randomized, open-label, phase III study of pembrolizumab (pembro) versus brentuximab vedotin (BV) in relapsed or refractory classic Hodgkin lymphoma (R/R cHL). Presented at the 2020 American Society of Clinical Oncology (ASCO) Virtual Scientific Program. May 29-31, 2020. abstract 8005.
- Ziepert M, et al: Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010; 28: 2373.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(2): 10-13.