Diabetes is associated with increased cardiovascular morbidity and mortality. Risk-stratified LDL cholesterol lowering is an important component of diabetes therapy and may improve clinical prognosis. In order to achieve the target values defined by the ESC, a stepwise therapy has proven effective. Statins continue to have an important role in lipid management. In addition, PCSK9 inhibitors and bempedoic acid are now available alongside ezetimibe.
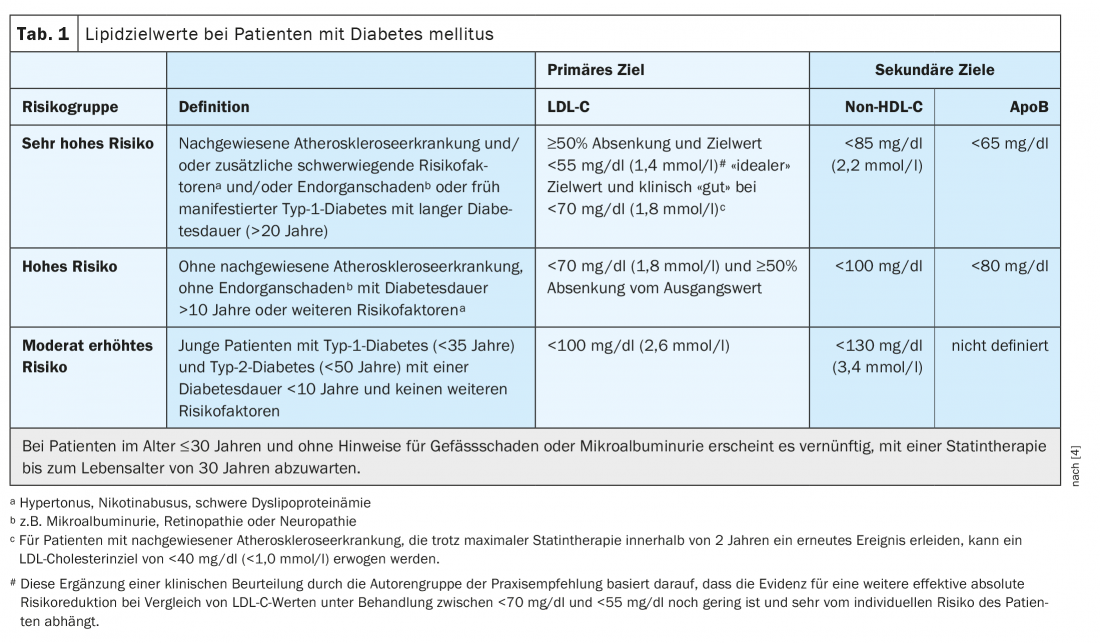
Patients with diabetes mellitus often have an increased cardiovascular risk. Risk-adapted lipid lowering based on the ESC-SCORE* (“systematic coronary risk estimation”) is an important countermeasure [1,2]. In patients at very high cardiovascular risk, the target is LDL cholesterol (LDL-C) <55 mg/dl or <1.4 mmol/l and a reduction of ≥50 percent, explains Prof. Dirk Müller-Wieland, MD, University Hospital RWTH Aachen (D) (Table 1) [3,4]. The primary goal is to reduce LDL cholesterol, but levels of non-HDL cholesterol (total cholesterol minus HDL cholesterol) are also relevant, as emphasized by the speaker, former president of the German Diabetes Association. The difference of non-HDL cholesterol to LDL cholesterol should not exceed 30 mg/dl (Table 1) [3,4]. Pathophysiologically, the activity of the LDL receptor in the liver is a central factor and key to therapeutic reduction of atherogenic lipoproteins and cardiovascular risk [5]. Characteristic of highly atherogenic diabetic dyslipidemia are hypertriglyceridemia, increased remnant particles, small dense LDL (“small dense”-LDL), and low HDL cholesterol. Insulin deficiency or insulin resistance leads to disinhibition of lipolysis and consequent increase in free fatty acids, which enter the liver in a concentration-dependent manner and are incorporated into triglyceride-rich lipoproteins (VLDL) under insulin [5]. “The more intensively you activate the LDL receptor – and one indicator is, of course, LDL cholesterol lowering – the more you also lower the atherogenic cholesterol content in these triglyceride-rich lipoproteins,” Prof. Müller-Wieland explained. This is associated with a significantly lower cardiovascular risk, as shown by various studies, the speaker added. In the practice recommendations of the DDG for lipid therapy in diabetes mellitus as well as in the guidelines of other international professional societies, this is taken into account accordingly [3 .4].
* The ESC-SCORE is available as an online tool and as a point table and gives the absolute 10-year risk in percent for a fatal atherosclerotic event (including sudden cardiac death). [1,2]
Statins still “first-line” option
“Currently, the basis for lipid therapy is and remains statin therapy,” said Julia Brandts, MD, University Hospital RWTH Aachen [6]. There are robust data that statins in both secondary prevention and primary prevention lead to a significant risk reduction in placebo comparisons. For every 1 mmol/l reduction in LDL cholesterol, a relative cardiovascular risk reduction of about 20% can be achieved, the speaker said. This is true regardless of the patient’s baseline risk and which drug is used to achieve this cholesterol reduction. For patients with diabetes mellitus who are at high or very high cardiovascular risk, a reduction in LDL-C of at least 50 percent is desired, which is why atorvastatin (40 mg) or rosuvastatin (20 mg) in particular are used for statin treatment.
Ezetimibe, bempedoic acid, and PCSK9-i for therapy escalation.
If statin therapy alone is not sufficient to achieve individual target values, the addition of ezetimibe is the next escalation step, Dr. Brandts suggests [6]. Ezetimibe decreases the absorption of cholesterol from the small intestine by specifically binding to NPC1L1 (Niemann-Pick C-1 like 1-protein). The IMPROVE-IT trial demonstrated that ezetimibe (10 mg/d) as an add-on to simvastatin (40 mg/d) reduced the rate of cardiovascular events more than statin monotherapy in patients randomized to this combination treatment within 10 days of the onset of an acute coronary syndrome, without increasing the rate of adverse events [7,8]. Another lipid-lowering therapeutic option is bempedoic acid, which inhibits cholesterol biosynthesis similarly to statins, but acts only in the liver, which is why myopathies are less common [4]. Decreased cholesterol biosynthesis results in a reduction of cholesterol in liver cells, which in the feedback mechanism results in more LDL receptors being expressed to remove LDL cholesterol from circulation. In the ongoing multicenter international CLEAR Outcomes study, cardiovascular end points are being followed up in high-risk patients over a 5-year period [13]. One can be curious about the results.
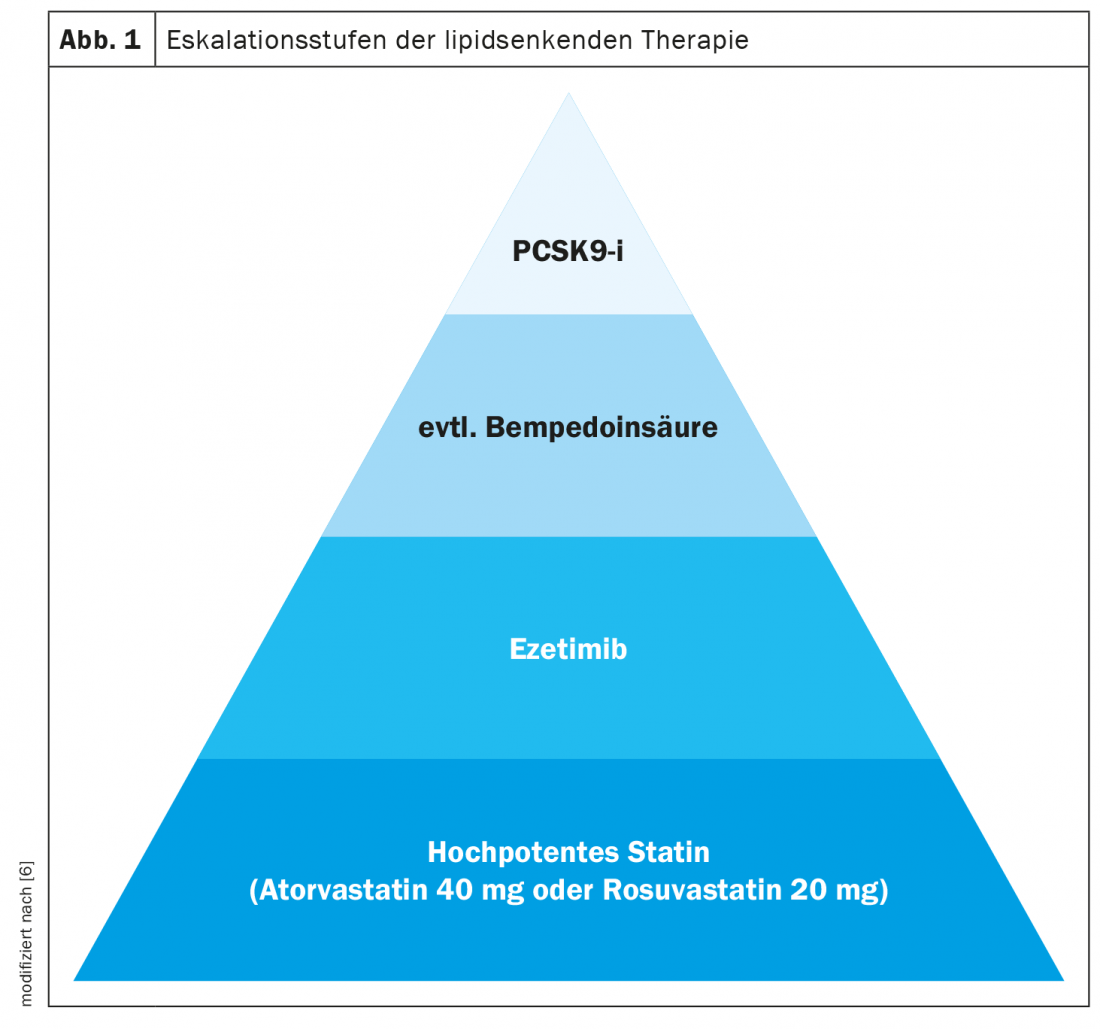
In high-risk patients who do not reach target levels despite the addition of ezetimibe and/or bempedoic acid, a PCSK9 inhibitor, for example alirocumab, evolocumab, or the siRNA therapeutic Inclisiran, can be used as a further escalation step. Alirocumab and evolocumab are monoclonal antibodies that are injected subcutaneously and selectively bind to the serine protease PCSK9 (proprotein convertase subtilisin-kexin type 9). Inclisiran – a “small interfering ribonucleic acid” (siRNA) – blocks the new synthesis of PCSK9, a serine protease that binds to LDL-C receptors on the surface of liver cells and promotes their degradation in hepatocyte lysosomes. Blocking the new synthesis of PCSK9 or its function increases the number of LDL receptors on the surface of hepatocytes. As a result, they bind more circulating LDL-C in liver cells and blood LDL-C levels decrease [9].
A relative risk reduction of 20% was achieved for evolocumab over a 36-month period in the FOURIER study. In the ODYSSEE-Outome Trial, treatment with alirocumab resulted in a 15% risk reduction compared with placebo, Dr. Brandts explained [6,10,11]. Inclisiran significantly reduced LDL cholesterol in patients with familial hypercholesterolemia or atherosclerotic cardiovascular disease in the ORION randomized trial program [12].
Outlook: promising further therapeutic options
“In the further clinical development of PCSK9 inhibitors, there are still the antisense oligonucleotides, the so-called ‘ASOS’,” said Dr. Brandts [6]. These are single-stranded molecules that bind and fragment mRNA due to their complementary structure. Of particular interest for the antisense oligonucleotides, she said, is that a greater than 60% reduction in LDL-C over 80 days can be achieved with once-monthly subcutaneous application [6]. In addition, an oral route of administration of a PCSK9 inhibitor was also tested for the first time. When administered daily, a 50% reduction in LDL-C had been achieved from day 7. Still in clinical development are small molecules called adnectins, Dr. Brandts reported. These also bind the extracellular circulating PCSK9 and thus act similarly to the monoclonal antibodies. “Again, there are initial phase II studies showing that a maximal reduction with a four-week applied dose of 300 mg achieved up to 76% reduction in LDL cholesterol,” she said [6].
Congress: Diabetology without borders
Literature:
- EAPC: HeartScore, www.heartscore.org, (last accessed 05.05.2022).
- ESC: SCORE: European High Risk Chart, www.escardio.org/static_file/Escardio/Subspecialty/EACPR/Documents (last accessed 05.05.2022).
- “Dyslipidemias in people with diabetes,” Prof. Müller-Wieland, MD, Diabetology without borders, Feb. 4-5, 2022.
- Parhofer KG, et al: Lipid therapy in patients with diabetes mellitus. www.deutsche-diabetes-gesellschaft.de (last accessed 05.05.2022).
- Müller-Wieland et al: Pathophysiological principles of dyslipoproteinemias. Dtsch Med Wochenschrift 2021; 146: e103-e112. DOI 10.1055/a-1516-2441
- “The Stepwise Therapy of Hypercholesterolemia – New Therapeutic Developments,” Julia Brandts, MD, Diabetology Limitless, Feb. 4-5, 2022.
- Giugliano RP, et al. (IMPROVE-IT): Circulation 2018 (10); 137(15): 1571-1582.
- DGK: Intensified lipid lowering with statin plus ezetimibe: who benefits most, 07/29/2019, www.kardiologie.org, (last accessed 05/05/2022).
- Lyko C, et al: PCSK9 inhibitors. Swiss Medical Forum 2017; 17 (45): 979-986.
- Sabatine MS, et al. (FOURIER): Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017; 376(18): 1713-1722.
- Schwartz GG, et al. (ODYSSEY): Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. NEJM 2018; 379(22): 2097-2107.
- Ray KK, et al: Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020; 382(16): 1507-1519.
- Ray KK, et al; CLEAR Harmony Trial. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N Engl J Med 2019; 380(11): 1022-1032.
HAUSARZT PRAXIS 2022; 17(5): 38-39
CARDIOVASC 2022; 21(2): 24-25