Type 1 diabetics with obesity in whom insulin therapy alone is insufficient for glycemic control may additionally benefit from oral treatment with an SGLT2 inhibitor. Recent study results show good results of add-on therapy. However, good adherence and monitoring for ketoacidosis risk are indicated.
(red) Worldwide, the annual incidence of type 1 diabetes mellitus is increasing by approximately 2-3% per year [1,2]. The disease is a result of autoimmune or nonimmune destruction of insulin-producing pancreatic β-cells and leads to an increase in glycated hemoglobin (HbA1c). Strict glycemic control may reduce the risk of microvascular (e.g., diabetic retinopathy, nephropathy, and neuropathy) and macrovascular (e.g., coronary artery disease and peripheral vascular disease) complications in patients with type 1 diabetes. Insulin remains the mainstay of drug treatment for type 1 diabetics. However, glycemic targets are not always achieved by insulin therapy alone [3]. Hypoglycemia and weight gain remain significant adverse events associated with insulin therapy. With the SGLT2 inhibitor dapagliflozin or the dual SGLT1-/SGLT2 inhibitor sotagliflozin, two additional options are now available that have shown good results, especially in diabetics with a BMI of 27 kg/m2 and above.
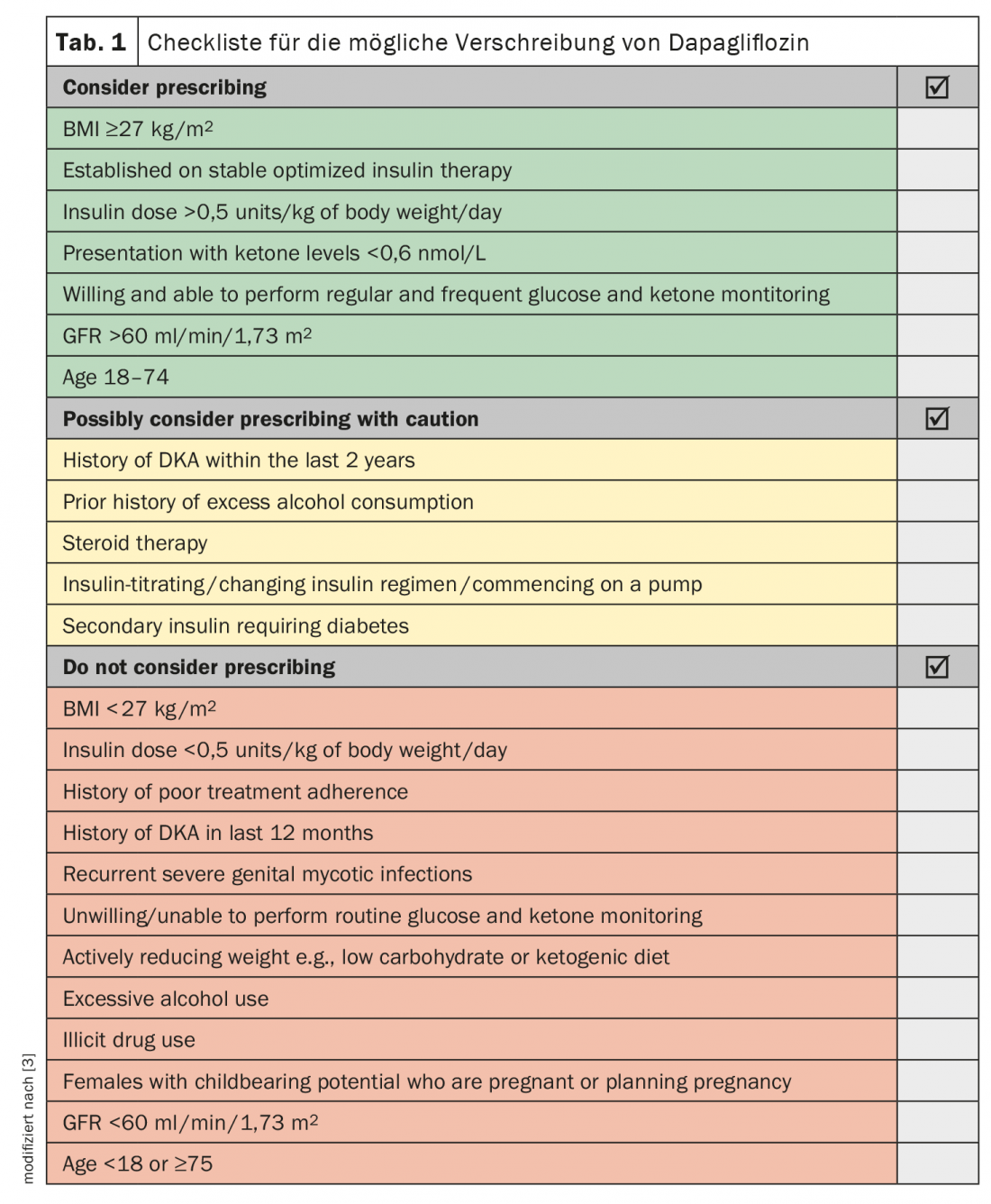
For dapagliflozin, the DEPICT clinical trial program has shown that as an add-on administration, it can support the achievement of glycemic goals in type 1 diabetic patients [3–5]. At week 52 in DEPICT1, placebo-adjusted changes in HbA1c and weight in the dapagliflozin 5/10 mg groups were -0.33/-0.%/–0,36% and -2.95 kg/-4.5 kg, respectively. Weight loss is likely to be important for many people with type 1 diabetes and may also limit subsequent cardiovascular risk. Patients best suited for dapagliflozin are likely to be those with a BMI ≥27 kg/m2 based on stable optimized insulin therapy and high insulin requirements (>0.5 units/kg body weight/day) (Table 1).
To improve glycemic control complementary to insulin therapy in adults with type 1 diabetes and a body mass index ≥27 kg/m2 who do not achieve adequate glycemic control despite optimal insulin therapy, the SGLT1/SGLT2 inhibitor sotagliflozin can also be used [6]. SGLT1 provides glucose uptake in the gastrointestinal tract and SGLT2 provides glucose reuptake in the kidneys. In the pivotal phase III Tandem study 3, 28.6% of type 1 diabetic patients dosed with sotagliflozin 400 mg once daily achieved HbA1c <7% (primary endpoint). In the placebo group, the figure was only 15.2%. However, attention should be paid to the risk of ketoacidosis, as significantly more ketoacidoses occurred (3% vs. 0.6%) [7].
Source: EASD 2020
Literature:
- Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ: Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010; 39(3): 481-497.
- Mayer-Davis EJ, Lawrence JM, Dabelea D, et al: Incidence trends of type 1 and type 2 diabetes in adolescents, 2002-2012. N Engl J Med. 2017; 376(15): 1419-1429.
- Evans M, et al: Optimising the Benefits of SGLT2 Inhibitors for Type 1 Diabetes. Diabetes Therapy 2020; 11: 37-52.
- Eliasson B: Adjunctive therapies in people with type 1 diabetes – beyond insulin. What is he future of type 1 diabetes treatment? Prof. Dr. Björn Eliasson, EASD Virtual Meeting, 23.09.2020
- Mathieu C, et al: Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes (the DEPICT-2 Study): 24-Week Results From a Randomized Controlled Trial. Diabetes Care 2018; 41(9): 1938-1946.
- MMW: Dual SGLT inhibitor receives marketing approval. MMW – Advances in Medicine 161, 58 (2019). https://link.springer.com/article/10.1007%2Fs15006-019-0564-y
- Garg SK, et al: Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 2017; 377: 2337-2348.
CARDIOVASC 2020; 19(4): 38 (published 9/12/20, ahead of print).