The new treatment options lead to higher therapeutic goals. This is also reflected in the current psoriasis guideline, which received an update last year. The determination of the severity is an important basis for the choice of the individually appropriate therapy. Here, the “upgrade criteria” must be taken into account. The majority of first-line treatments are conventional systemic therapeutics, but some biologics now also have a “first-line” label.
The great abundance of drug innovations in the indication area of moderate to severe psoriasis has raised the therapy goals to a new level: “We want to achieve extensive healing and this is really achievable with the drugs available today,” says Prof. Matthias Augustin, MD, Director at the Institute for Health Services Research in Dermatology and Nursing Professions, University Hospital Hamburg [1]. The clinical benchmark for psoriasis treatment today is freedom from skin manifestations and restoration of quality of life. The therapeutic approach is tailored to individual preferences (“shared decision making”). A look at the data shows that the objective severity, operationalized by means of the Psoriasis Area and Severity Index (PASI), as well as the losses in quality of life have decreased in recent years, according to Prof. Augustin. The 2021 psoriasis S3 line update discusses treatment algorithms and currently available systemic agents in detail [1,2].
Consider localization of skin lesions as well as quality of life
It is important that those patients who need systemic therapy have access to it, the expert stresses. In this context, the so-called “upgrade criteria” are significant: According to this, the classification as moderate to severe psoriasis is not limited to cases in which the BSA or PASI and DLQI values are above 10, but the following criteria are alternatively decisive: pronounced disease of visible areas, pronounced disease of the scalp, disease of the genital area, disease of the palms and soles, onycholysis or onychodystrophy of at least two fingernails, itching and associated scratching, presence of therapy-resistant plaques [2]. When selecting the appropriate systemic therapy, the guideline recommends considering the following factors in addition to efficacy and safety: Time to onset of action, comorbidity, and individual patient factors/preferences.
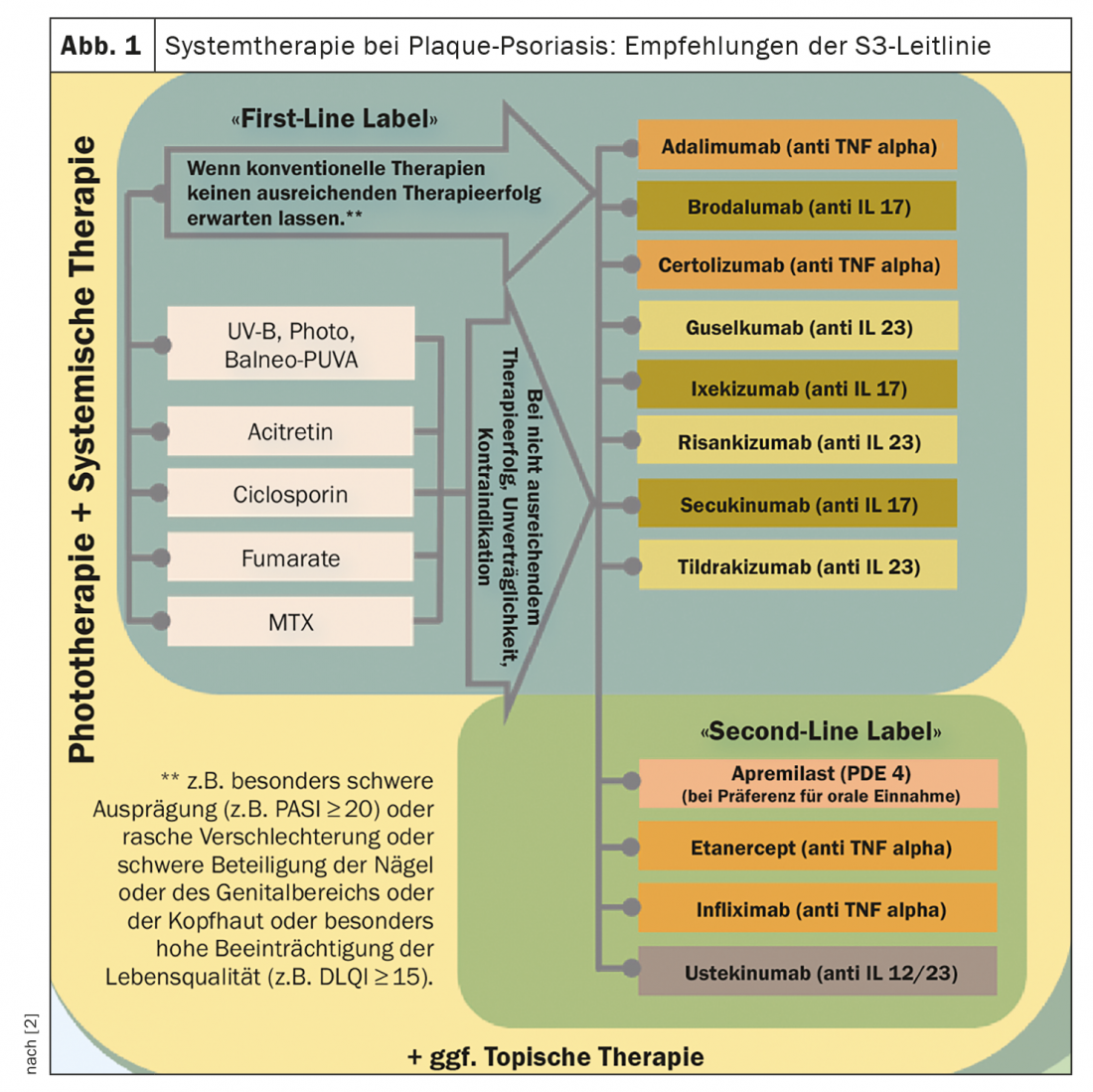
Biologics as first-line therapy – a look at registry data.
According to Prof. Augustin, an analysis of data from the PsoBest registry* shows that a biologic is used as a “first-line” treatment in about 30% of patients with moderate to severe psoriasis in Germany [1]. This share has risen noticeably since 2015. Secukinumab is the most commonly used, accounting for 29%, followed by guselkumab, which is used as a first-line systemic therapeutic in 20% of cases [1]. About two-thirds receive conventional systemic therapeutics as a “first systemic drug,” with dimethyl fumarate and methotrexate being by far the most common. The recommendations made in the guideline (Fig. 1) are based on empirical evidence as well as on the consensus-agreed clinical experience of the expert panel [2]. The selection of the appropriate systemic therapy for special clinical situations or for psoriasis patients with comorbidities is discussed and highlighted in detail in the guideline.
* The Psoriasis Registry PsoBest collects data from patients with psoriasis and psoriatic arthritis who are prescribed a biologic or other systemic therapy for the first time.
Congress: Update Inflammatory Skin Diseases
Literature:
- Augustin M: Update Psoriasis: What is important for the practice, Prof. Dr. med. Matthias Augustin, 5th Lübeck Update Inflammatory Skin Diseases, 05.03.2022.
- Nast A, et al: German S3 guideline on the therapy of psoriasis vulgaris, 2021. J Dtsch Dermatol Ges, www.awmf.org, (last accessed 24.05.2022).
DERMATOLOGIE PRAXIS 2022; 32(3): 20