Among the approximately 200 abstracts on thoracic oncology at this year’s ASCO Annual Meeting , exciting news was found especially on non-small cell lung cancer (NSCLC). With immunotherapeutic options in increasingly earlier lines of treatment and new targeted therapies in the presence of a driver mutation, some doors may soon open.
Sotorasib, amivantamab/lazertinib, and patritumumab-deruxtecan-these drug names could potentially play important roles in thoracic oncology in the future. All four agents are currently being investigated for use in metastatic NSCLC with Driver mutation. While the small molecule inhibitor sororasib has the previously untargeted KRAS, amivantamab/lazertinib and patrimumab-deruxtecan find their use in patients with an activating EGFR mutation after failure of first-line therapy. In addition to these new agents, this year’s ASCO Annual Meeting focused on one question in particular: When is the optimal time for immunotherapy in NSCLC? Current data suggest that this may be earlier than previously thought. Namely, already neoadjuvant.
Targeting driver mutations
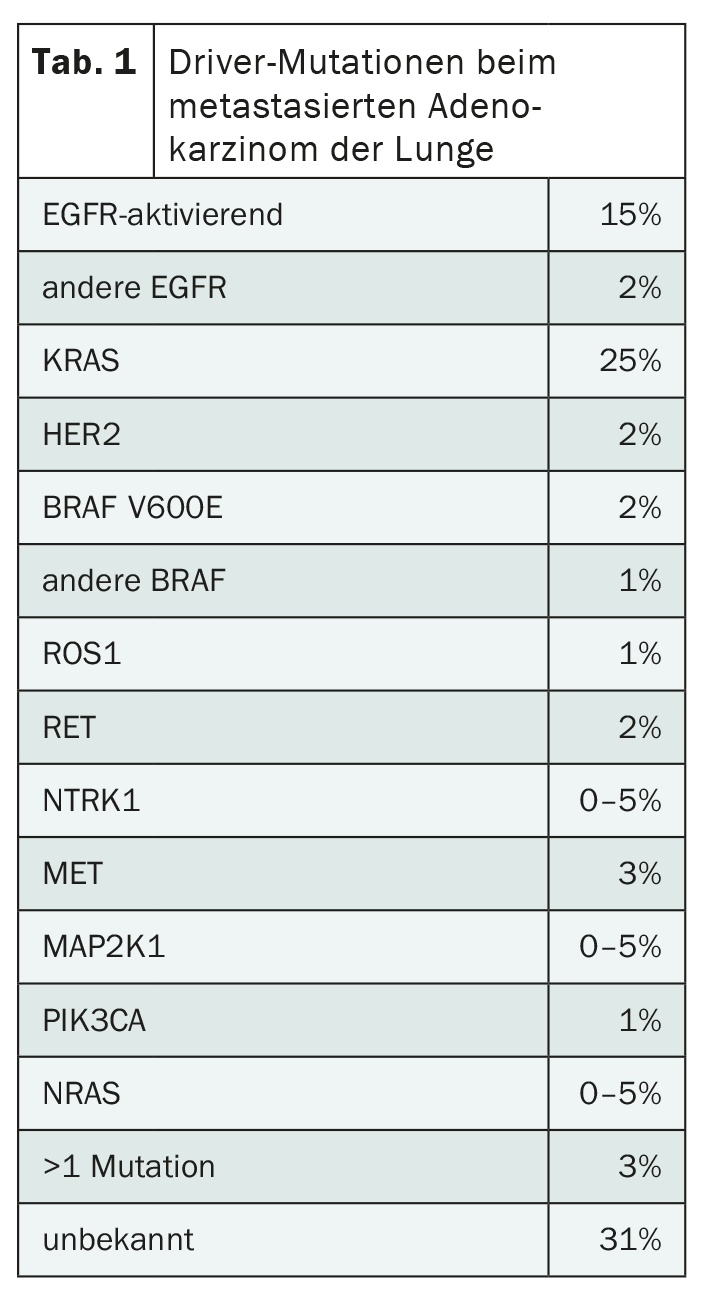
The development of targeted therapies has led to a sustained improvement in the prognosis of NSCLC over the past years. And yet there is still room for improvement, because resistance to modern treatment methods often occurs. In addition, driver mutations exist that cannot yet be targeted (Tab. 1) . One such mutation is KRAS, which affects a good quarter of all patients with adenocarcinoma of the lung. It is therefore the most common driver mutation – and unlike the others, it occurs more frequently in smokers. Apart from NSCLC, it also occurs in other entities. For example, KRAS is mutated in a large proportion of pancreatic and colon cancers, among others.
Sotorasib, also known as AMG510, was developed as the first specific therapy targeting KRAS. This is an oral, irreversible and selective KRAS p.G12C inhibitor. The p.G12C mutation accounts for about half of all KRAS mutations – and accordingly affects about 13% of all patients with adenocarcinoma of the lung. The first promising data from the CodeBreak 100 study were already presented last year. These were confirmed at this year’s ASCO Annual Meeting, and for the first time, overall survival data were also published in the Phase II study. We studied 126 patients with metastatic bronchus adenocarcinoma and KRAS p.G12C mutation who experienced progression after standard first-line treatment. Stable brain metastases did not represent an exclusion criterion. The study participants received once-daily suterasib until disease progression. This showed an objective response rate (ORR) of 37.1% with a disease control rate (DCR) as high as 80.6%. This includes all patients with stabilization of the tumor. Response lasted a median of 11.1 months, with a median progression-free survival (PFS) of 6.8 months and a median overall survival (OS) of 12.5 months. The only slow decline of the PFS curve, reflecting stabilization over time, is considered particularly promising. No fatal adverse events occurred, the dose had to be adjusted – mostly due to liver injury – in 22.2% of the study participants, and 7.1% discontinued therapy due to adverse drug reactions. Overall, there was good tolerability with mostly well-controlled, predominantly gastrointestinal side effects. A final assessment is certainly not yet possible based on this phase II study, but sororasib represents a new therapeutic option in the prognostically unfavorable setting of progressive metastatic NSCLC with KRAS p.G12C mutation that is likely to be superior to standard second-line therapy. According to the current data, treatment with soterasib prolongs PFS by four months and OS by five months compared to docetaxel therapy – with a significantly higher response rate and better tolerability.
New agents are also in the pipeline for EGFR-mutated tumors. Thus, two therapeutic regimens are currently being investigated after failure of first-line therapy in metastatic NSCLC with activating EGFR mutation. In this situation, no adequate targeted therapy is available so far, and the standard treatment consists of chemotherapy with carboplatin and pemetrexed. Activating EGFR mutations are the second largest group of driver mutations in adenocarcinoma of the lung, affecting approximately 15% of patients. Development of resistance during first-line treatment with EGFR tyrosine kinase inhibitor is a common problem in this patient group. Various mechanisms and mutations cause the EGFR tyrosine kinase inhibitor – usually osimertinib – to eventually stop working. Currently, the median PFS with first-line therapy is approximately 1.5 years. Both regimens currently being tested for second-line therapy in clinical trials are independent of the exact resistance mutation, which could significantly simplify everyday clinical practice in the future.
On the one hand, a trial in 45 patients is testing the drug combination of amivantamab, a bispecific antibody directed against EGFR and MET, and lazertinib, a third-generation EGFR tyrosine kinase inhibitor, after failure of osimertinib treatment. Results presented at the ASCO Annual Meeting were encouraging: the objective response rate was 36%, with a median duration of response of 9.6 months and a median PFS of 4.9 months. On the other hand, the antibody-drug conjugate patritumumab-deruxtecan is currently being evaluated for use in second-line treatment of metastatic NSCLC. The corresponding study includes 57 participants whose NSCLC progressed on EGFR tyrosine kinase inhibitor and chemotherapy. Here, the objective response rate was 39% with a median response duration of 7 months and a median PFS of 8.2 months. Response was independent of HER3 expression and EGFR tyrosine kinase inhibitor used in first-line treatment. Furthermore, it did not matter whether brain metastases were present. Patritumumab-deruxtecan consists of an antibody against HER3, a linker and a topoisomerase I inhibitor as payload.
Non-resectable NSCLC: maintenance therapy with durvalumab.
In addition to these new agents, the already approved checkpoint inhibitor durvalumab was also a hot topic at the ASCO Annual Meeting. This already represents the standard of care for locally advanced, unresectable NSCLC without progression after platinum-based radiochemotherapy [1]. And rightly so, as the 5-year data from the Phase III PACIFIC study suggest. The randomized-controlled trial included 713 patients regardless of PD-L1 status who received durvalumab or placebo for a maximum of one year for maintenance therapy after radiochemotherapy. There was also a clear benefit of durvalumab treatment at 60 months with an OS hazard ratio of 0.72 and a PFS hazard ratio of 0.55. Overall survival at five years in the intervention group was 42.9%, compared with 33.4% in the placebo group. However, the question of whether the early use of immunotherapy is worthwhile cannot be conclusively answered on the basis of this study, because a crossover was not approved. Thus, the survival benefit could be due to the administration of durvalumab per se and not to the early timing. Nonetheless, the longer-term data from the PACIFIC trial are a strong indication for maintenance therapy with durvalumab in non-resectable, locally advanced NSCLC.
Adjuvant or neoadjuvant immunotherapy?
Checkpoint inhibitor therapy may also take on a more important role in resectable tumors in the future. Two Phase III studies presented at the ASCO Annual Meeting are currently taking a closer look at these. While the IMpower010 trial is looking at immunotherapy in the adjuvant setting, the Checkmate 816 trial is looking at neoadjuvant administration of the checkpoint inhibitor nivolumab. This is an important topic, because even operable NSCLC has a high systemic and locoregional recurrence rate, which is probably due to the early development of micrometastases. Therefore, adjuvant chemotherapy was already introduced 20 years ago in stages IC-III after complete resection. However, this can only increase the healing rate by 5-10% after five years. Earlier use of immunotherapy could increase the efficacy of adjuvant or neoadjuvant systemic therapy and possibly improve surgical outcomes. This is because, according to findings from the Checkmate 816 study, preoperative checkpoint inhibitor administration leads to a significant increase in the surgical rate, particularly in more advanced tumor stages, as primary progression can be prevented more efficiently. In addition, neoadjuvant therapy with nivolumab showed more minimally invasive surgeries, fewer conversions, fewer pneumonectomies, and significantly shorter surgery times. Thus, the concern that surgery might be more difficult after neoadjuvant immunotherapy due to increased fibrosis was refuted in this study. The rate of R0 resections was approximately 80% both with and without neoadjuvant nivolumab administration. Neoadjuvant checkpoint inhibitor administration is also supported by preclinical data suggesting that the efficacy of immunotherapy can be enhanced by preoperative administration. This could be due to the fact that a higher initial tumor burden can support treatment and cushion the postoperative immunosuppressed state.
Even apart from surgical outcomes, the data published to date from the Checkmate 816 trial paint a positive picture of adding neoadjuvant nivolumab to chemotherapy for newly diagnosed stage IB-IIIA NSCLC without EGFR or ALK mutation. Notably, there was a significantly higher rate of pathologically complete remission at the surgical resection site: 24% in the intervention group and 2.2% in the control arm. This effect was independent of tumor stage. Those patients with PD-L1 expression of ≥50% benefited the most, but the rate of pathologic complete remissions increased significantly with nivolumab therapy even in PD-L1-negative tumors.
The option of adjuvant immunotherapy using atezolizumab is being evaluated in the IMpower010 trial in over 1000 patients with completely resected stage IB-IIIA NSCLC. Study participants received 1-4 cycles of platinum-based chemotherapy after surgery, followed by 16 cycles of atezolizumab or Best Supportive Care. Data on the primary endpoint of disease-free survival (DFS) were presented at the ASCO Annual Meeting. Patients with high PD-L1 expression appear to benefit most from therapy after three years according to this analysis, while no statistically significant difference was demonstrated in the overall population. Subgroup analysis suggests that patients without PD-L1 expression and those with EGFR- or ALK-mutated tumors are unlikely to benefit from additional adjuvant atezolizumab treatment, whereas the beneficial effect was significant in the other subgroups. There, the DFS curve diverges after only three to six months – an effect that persists even after discontinuation of adjuvant therapy. What must not be neglected, especially in the adjuvant setting, is the sometimes lethal side effect profile of checkpoint inhibitors. Thus, four study participants died from adverse drug reactions. Overall, adjuvant atezolizumab administration in stages II-IIIA with PD-L1 expression ≥50% is considered reasonable based on the study results to date.
Whether neoadjuvant or adjuvant, immunotherapy is likely to become an integral part of first-line treatment in resectable NSCLC. At present, it is still difficult to assess in which combinations and under which conditions use is most effective, and this will have to be seen over the next few years. We remain curious.
Source: presentation “ASCO 2021: Highlights Lung Tumors”, Laetitia Mauti, Forum for Continuing Medical Education WebUp Expert Forum “Update Oncology & Hematology: Post ASCO 2021” on June 26, 2021.
Literature:
- Drug information from swissmedic Swiss Agency for Therapeutic Products. www.swissmedicinfo.ch (last accessed on 01.07.2021)
InFo ONCOLOGY & HEMATOLOGY 2021; 9(4): 22-24 (published 9/20-21, ahead of print).
InFo PNEUMOLOGY & ALLERGOLOGY 2021; 3(4): 24-26.