ANZEIGE

NOW AVAILABLE! 7-Year MonarchE Results
Overall Survival and IDFS Outcome
In node-positive, high-risk*, HR+, HER2- EBC, only Verzenios® is proven to increase survival and deliver long-lasting protection from recurrence with 2 years of treatment, so you can help give her a future of opportunity†,1-3
If you missed the events, you can watch the full recordings here!
*Verzenios in combination with ET (AI or tamoxifen) is indicated for the adjuvant treatment of adult patients with HR+, HER2−, node-positive EBC at a high risk of recurrence. High-risk EBC in Cohort 1 was defined as patients who had ≥4 positive nodes or 1–3 positive nodes and tumors that were ≥5 cm, histological Grade 3—or both.5 † A statistically significant OS benefit was seen in the ITT population. Following regulatory consultation at the primary IDFS analysis, the OS analysis plan was amended to increase final events from 390 to 650 to ensure ≥5 years’ follow-up. Verzenios is approved for Cohort 1 (91% of ITT); OS analysis in this subpopulation was not powered or alpha-controlled.1,3
References: 1. Johnston S, et al. Overall Survival with Abemaciclib in Early Breast Cancer, Annals of Oncology (2025), doi: https://doi.org/10.1016/j.annonc.2025.10.005. 2. Verzenios. Summary of Product Characteristics. www.swissmedicinfo.ch 3. Rastogi P et al. J Clin Oncol. 2024;42(9):987-93. 4. Hortobagyi GN et al. Ann Oncol. 2025;36(2):149-57.
Indication2
Verzenios® is a CDK4/6 inhibitor indicated for:
- The adjuvant treatment of HR+, HER2−, node-positive early breast cancer at high risk* of relapse based on lymph node status, primary tumour size and tumour grade.
- in combination with endocrine therapy (tamoxifen or an aromatase inhibitor) in adult female patients at high risk of recurrence*
- In pre- or perimenopausal women, aromatase inhibitor endocrine therapy should be combined with an LHRH agonist Indication5
In EBC, Swiss approval was granted based on the MonarchE Cohort 1, which represents 91% of the ITT population**.2,3
Cohort 1 inclusion criteria:3
- 1 to 3 positive axillary lymph nodes and tumor size ≥ 5 cm OR
- 1 to 3 positive axillary lymph nodes and histological tumor grade 3 OR
- ≥ 4 positive axillary lymph nodes
Verzenios® is a CDK4/6 inhibitor indicated for:
- The treatment of HR+, HER2- locally advanced or metastatic breast cancer
- Verzenios® in combination with an aromatase inhibitor as primary ET or in combination with fulvestrant in patients who have previously under-gone ET
- The treatment of postmenopausal HR+, HER2- locally advanced or metastatic breast cancer as monotherapy after disease progression following hormone therapy and one or two chemotherapy regimens, in cases of metastatic disease when chemotherapy is not indicated.
- In pre- or perimenopausal women, endocrine therapy should be combined with an LHRH agonist
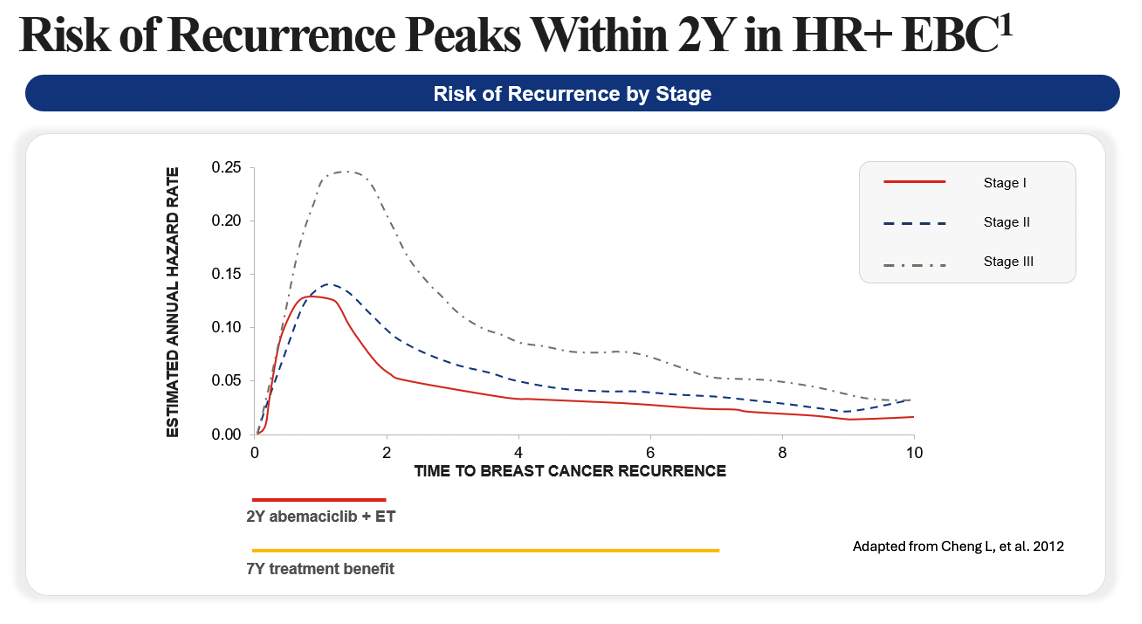
Risk of recurrence in EBC
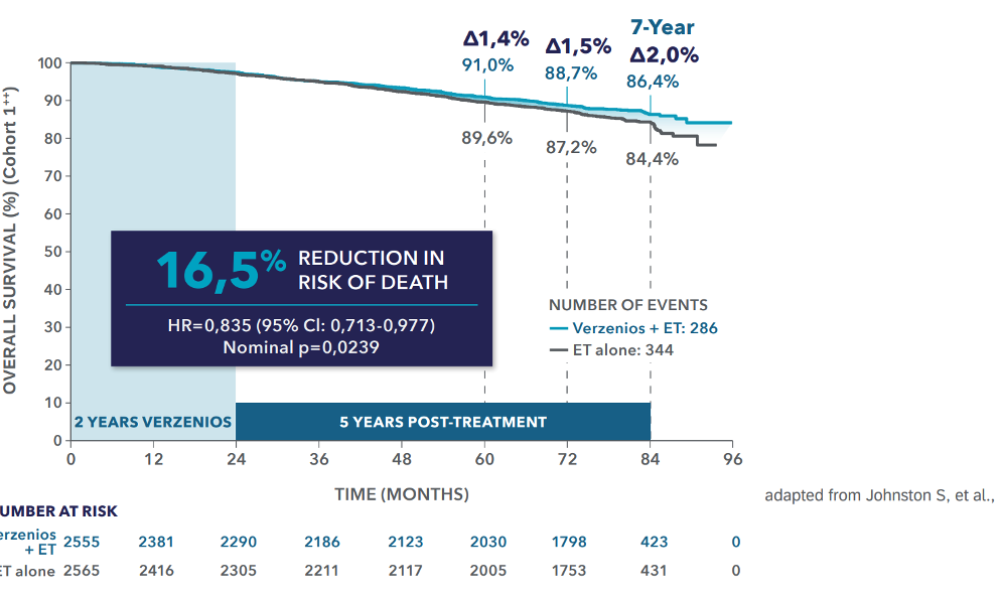
Breakthrough overall survival data: Verzenios® in combination with AI or tamoxifen increases survival†,1
Efficacy (Cohort 1**) │ Overall survival benefit at 7 years1
Verzenios® is the only CDK4/6 inhibitor with a proven overall survival benefit in the curative setting and 2 years of treatment†, *,1,4
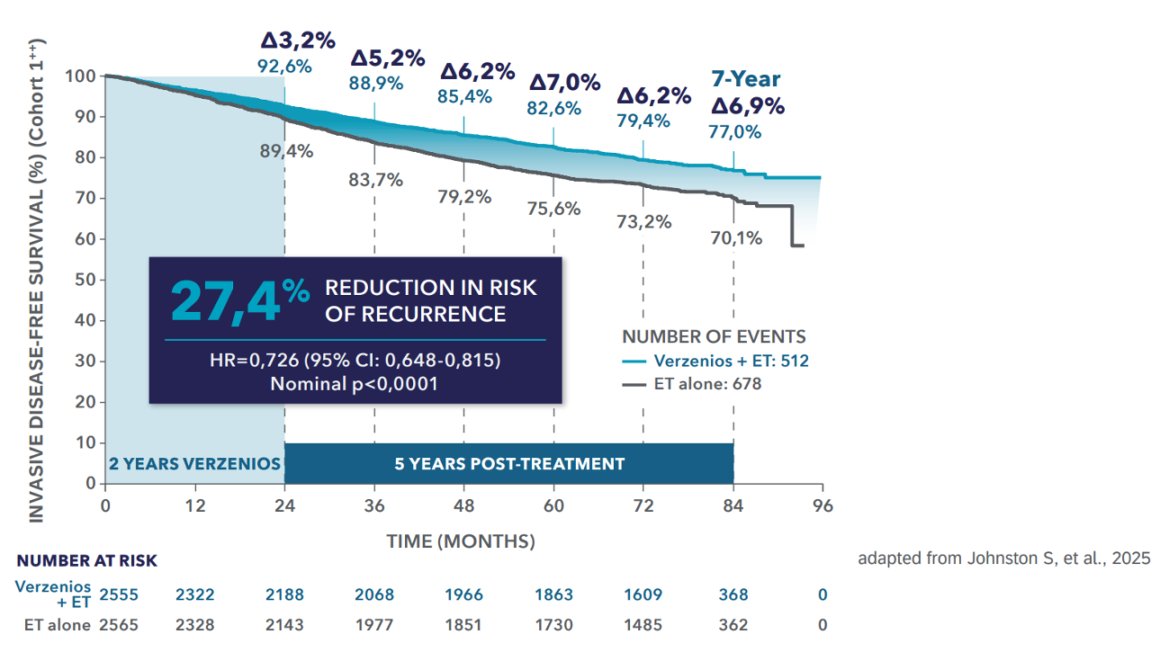
Efficacy (Cohort 1**) │ IDFS benefit at 7 years1
Verzenios® delivers 7 years of sustained IDFS benefit with 2 years of treatment*,1
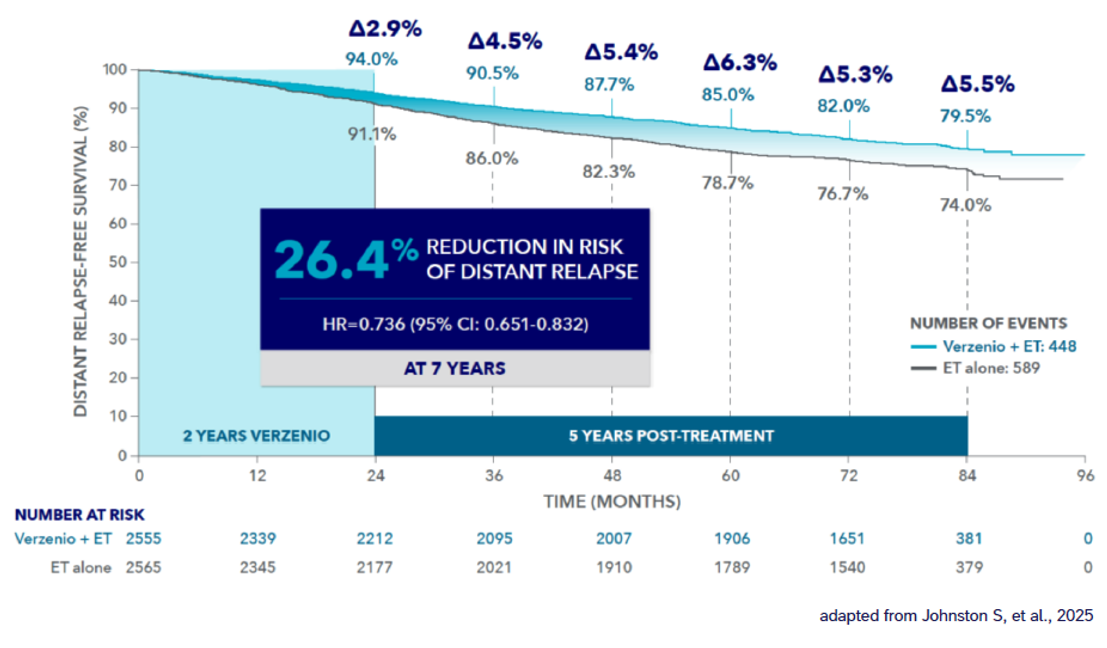
Verzenios® delivers long-lasting protection – reducing the risk of recurrence to incurable metastatic disease1*
DRFS benefit sustained at 7 years (Cohort 1**)1
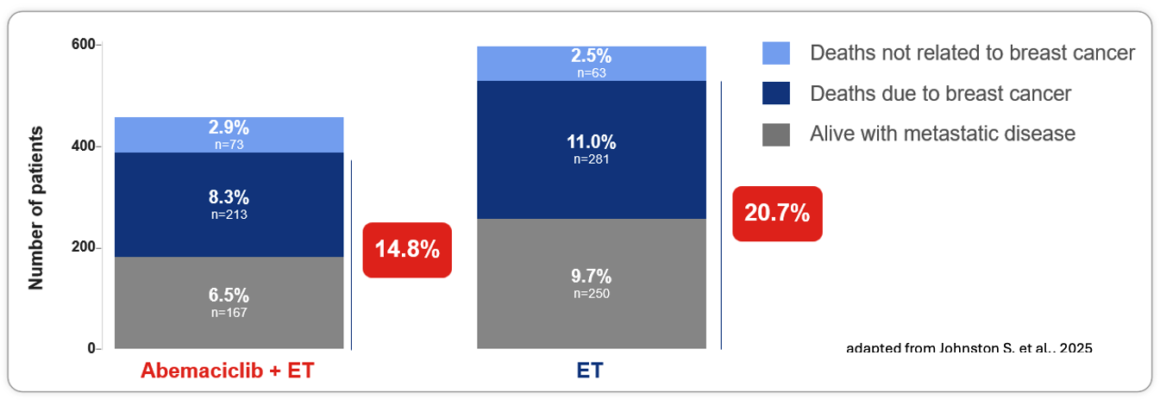
~30% Fewer Patients in the Abemaciclib Arm Developed and Are Living With Metastatic Disease (Cohort 1**)1
References: 1. Johnston S, et al. Overall Survival with Abemaciclib in Early Breast Cancer, Annals of Oncology (2025), doi: https://doi.org/10.1016/j.annonc.2025.10.005. 2. Verzenios. Summary of Product Characteristics. www.swissmedicinfo.ch 3.Rastogi P et al. J Clin Oncol. 2024;42(9):987-93. 4. Hortobagyi GN et al. Ann Oncol. 2025;36(2):149-57. 5. Cheng L et al. Cancer Epidemiol Biomarkers Prev. 2012;21(5):800-9.
*Verzenios® in combination with ET (AI or tamoxifen) is indicated for the adjuvant treatment of adult female patients with HR+, HER2−, node-positive EBC at a high risk of recurrence. High-risk EBC in Cohort 1 was defined as patients who had ≥4 positive nodes or 1–3 positive nodes and tumors that were ≥5 cm, histological Grade 3—or both.5 †A statistically significant OS benefit was seen in the ITT population. Following regulatory consultation at the primary IDFS analysis, the OS analysis plan was amended to increase final events from 390 to 650 to ensure ≥5 years’ follow-up. Verzenios is approved for Cohort 1 (91% of ITT); OS analysis in this subpopulation was not powered or alpha-controlled.1-3 ‡Defined as “reduction in risk of recurrence over time”.2,3 §High-risk features are defined as ≥4 positive lymph nodes or 1 to 3 positive lymph nodes with ≥1 of the following: Grade 3 disease, tumor size ≥5 cm. Patients without these high-risk features had stage I-III EBC that did not meet these criteria (node-negative, or node-positive with 1 to 3 positive nodes and tumor size <5 cm, and histological Grade <3).7 **In Cohort 1 0.6 % of patients were male. Male population is excluded for the Swiss approval.
Treatment Adherence and Dose Adjustments
A significant proportion of patients required dose reductions during the 2-year treatment period. Importantly, efficacy was maintained even in those who received reduced doses, highlighting the flexibility of the regimen and its tolerability in real-world settings (Cohort1**).5
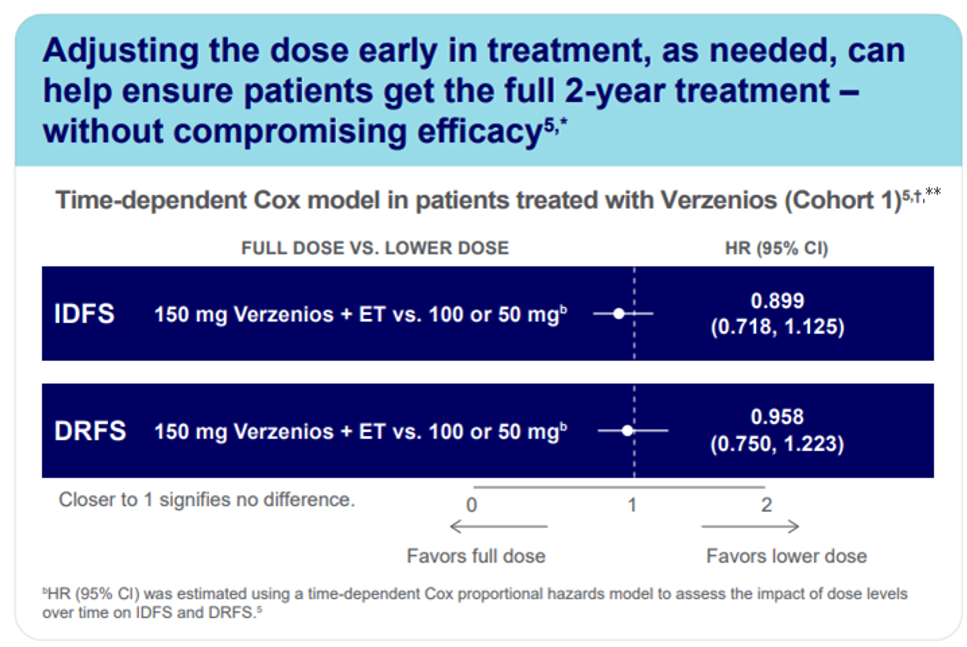
*44% of patients received a dose reduction to help them remain on treatment.2,5 † Cohort 1 included patients who had ≥4 positive nodes or 1-3 positive nodes and tumors that were ≥5 cm, histological Grade 3, or both.5 3 **In Cohort 1 0.6 % of patients were male. Male population is excluded for the Swiss approval.
References: 1. Johnston SRD et al. Lancet Oncol. 2023;24(1):77-90. 2. Verzenios Summary of Product Characteristics. www.swissmedicinfo.ch 3. Rugo HS et al. Ann Oncol. 2022;33(6):616-27. 4. Johnston SRD et al. J Clin Oncol. 2020;38(34):3987-98. 5. Goetz MP et al. NPJ Breast Cancer. 2024;10(1):34.
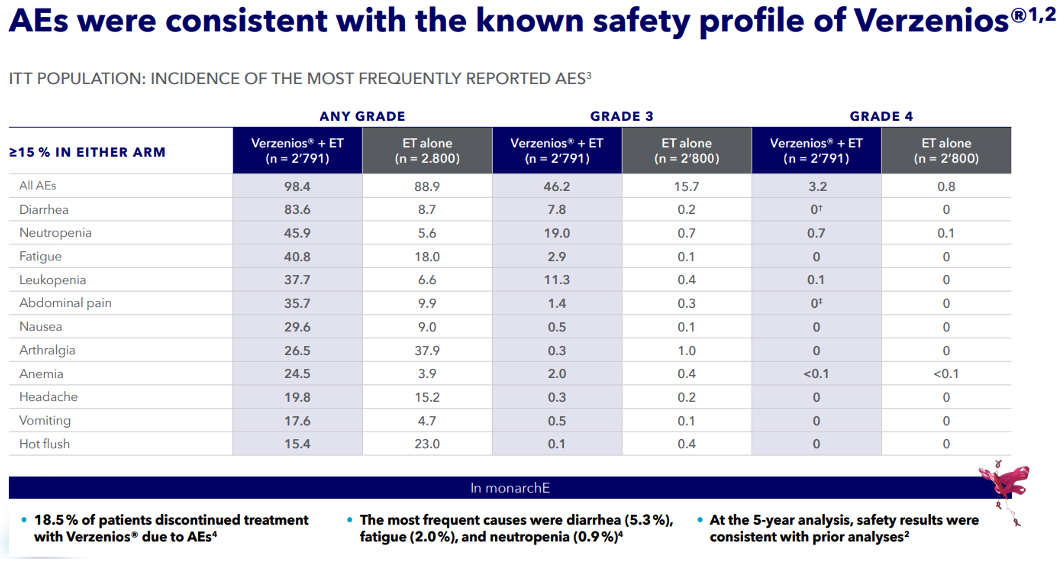
Safety Profile
1. Verzenios® Summary of Product Characteristic. www.swissmedicinfo.ch. 2. Rastogi P, et al. Adjuvant Abemaciclib Plus Endocrine Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative, High-Risk Early Breast Cancer: Results From a Preplanned monarchE Overall Survival Interim Analysis, Including 5-Year Efficacy Outcomes. J Clin Oncol. 2024 Mar 20;42(9):987-993. 3. Johnston SRD, et al. Abemaciclib plus Endocrine Therapy for Hormone Receptor-Positive, HER2-negative, Node-Positive, High-Risk Early Breast Cancer (monarchE): Results from a Preplanned Interim Analysis of a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2023 Jan;24(1):77-90. 4. Rugo HS, et al. Adjuvant Abemaciclib Combined with Endocrine Therapy for High-Risk Early Breast Cancer: Safety and Patient-Reported Outcomes from the monarchE Study. Ann Oncol. 2022 Jun;33(6):616-627.
AEs: Adverse events
Want to learn more?

Eli Lilly (Suisse) SA, Chemin des Coquelicots 16,P.O. 580, CH-1214 Vernier
PP-AL-CH-0775/01.2026
Abbreviations: AE=adverse event; AI=aromatase inhibitor; CDK4/6i=cyclin-dependent kinase 4/6 inhibitor; CI=confidence interval; DRFS=distant relapse-free survival; EBC=early breast cancer; EMA=European Medicines Agency; ET=endocrine therapy; HER2−=human epidermal growth factor receptor 2-negative; HR+=hormone receptor-positive; HR=hazard ratio; IDFS=invasive disease-free survival; KM=Kalpan-Meier; QoL=quality of life.
Verzenios® (abemaciclib) film-coated tablets I: Temporarily authorized indication: Adjuvant treatment in combination with endocrine therapy (ET) of adult women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive early breast cancer at high risk of relapse based on lymph node status, primary tumour size and tumour grade. In pre- or perimenopausal women, aromatase inhibitor endocrine therapy should be combined with a LHRH agonist. Permanent Indication: Treatment of postmenopausal women with HR+, HER2- locally advanced or metastatic breast cancer: in combination with an aromatase inhibitor as initial endocrine-based therapy or in combination with fulvestrant in women who have received prior endocrine therapy. As monotherapy following disease progression after endocrine therapy and one or two chemotherapy regimens in the metastatic setting, when chemotherapy is not suitable. In pre- or perimenopausal women combined with a LHRH-agonist. P: The recommended dose is 150 mg twice daily when used in combination with endocrine therapy and as a single agent 200 mg twice daily.CI: Hypersensitivity. W/P: For women who have not received any (neo-) adjuvant chemotherapy before, the available data is limited, because only 2% of women in the monarchE study were included. No statistically significant overall survival benefit from abemaciclib was shown at temporary approval. Its impact on survival compared to later relapse treatment, or on subsequent advanced cancer therapies, remains unassessed. Neutropenia, infections, Interstitial Lung Disease (ILD)/Pneumonitis, diarrhoea, increased aminotransferases, venous thromboembolism and arterial thromboembolic events occurred. Contains lactose. Verzenios can have an influence on the ability to drive and use machines. IA: Abemaciclib and its circulating active metabolites resulted in a down-regulation of CYPs’ mRNA, including CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2D6, and CYP3A4 in vitro. In a clinical drug interaction study in women with cancer, multiple doses of abemaciclib did not result in clinically meaningful changes in the pharmacokinetics of CYP1A2 (caffeine), CYP2C9 (S-warfarin), CYP2D6 (dextromethorphane) and CYP3A4 substrates (midazolam). The clinical effects of CYP2C8 and CYP2B6 down regulation are unknown. Abemaciclib is a substrate of CYP3A4, time dependent changes in pharmacokinetics of abemaciclib as a result of autoinhibition of its metabolism were not observed. When strong CYP3A4 inhibitors such as for example clarithromycin, itraconazole, ketoconazole, lopinavir/ritonavir, posaconazole or voriconazole need to be co-administered, the dose of abemaciclib should be reduced. Caution and monitoring of toxicity is recommended during concomitant treatment with sensitive substrates of P-gp or BCRP that have a narrow therapeutic index, such as digoxin and dabigatran. Sensitive substrates of P-gp or BCRP that do not have a narrow therapeutic index such as pitavastatin, pravastatin and rosuvastatin may be used with caution. Abemaciclib and its major active metabolites inhibit the renal transporters OCT2, MATE1, and MATE2-K at concentrations achievable at the approved recommended dosage. Pr/L: There are no data from the use of abemaciclib in pregnant women. Animal studies have shown reproduction toxicity. Therefore, Verzenios should not be used during pregnancy and in women of childbearing potential without use of contraception, unless this is absolutely necessary. If Verzenios is used during pregnancy or if a patient gets pregnant during therapy, the patient should be advised of the potential risk for the fetus. It is unknown whether abemaciclib is excreted in human milk. A risk to newborns/infants cannot be excluded. Women should not breast-feed during treatment with abemaciclib and for at least up to 3 weeks after last administration of abemaciclib. ADR: Very common: Infections, neutropenia, anemia, leukopenia, thrombocytopenia, decreased appetite, dysgeusia, dizziness, diarrhoea, nausea, abdominal pain, vomiting, stomatitis, alopecia, rash, pruritus, fatigue, pyrexia, increased ASAT/ALAT. Common: Lacrimation increase, venous thromboembolism, lymphopenia, Interstitial lung disease/pneumonitis, dry mouth, dry skin, muscular weakness. P: 50 mg, 100 mg, 150 mg, 200 mg: 28 and 56 film-coated tablets. Dispensing category A.. Consult www.swissmedicinfo.ch for further information. Eli Lilly (Suisse) SA, ch. des Coquelicots 16, CP 580, 1214 Vernier (GE). V12-2023
Dieser Inhalt ist werbewirksam und wurde von Eli Lilly (Suisse) SA entwickelt und gesponsert.
Er ist nur für medizinisches Fachpersonal bestimmt. Bitte kopieren oder übertragen Sie ihn nicht.
Copyright© 2025. Alle Rechte vorbehalten.
Eli Lilly (Suisse) SA, Chemin des Coquelicots 16,1214 Vernier, Schweiz.