At this year’s congress of the European Association of Dermato-Oncology (EADO), current recommendations for the management of cutaneous squamous cell carcinoma (cSCC) were outlined. The first-line therapy remains complete excision with histologic incision margin control. Adjuvant radiotherapy may be considered depending on the risk of recurrence. In advanced non-resectable cSCC, immune checkpoint inhibition is considered first-line therapy. Chemotherapeutic agents or EGFR inhibitors may be used if contraindications exist. Follow-up should be risk-adapted and include lymph node ultrasonography in high-risk patients.
Squamous cell carcinoma of the skin – a malignant neoplasm of epidermal keratinocytes – is the most common malignant skin tumor in humans after basal cell carcinoma. Prof. Alexander J. Stratigos, MD, Department of Dermatology-Venereology, Andreas Sygros Hospital, National and Kapodistrian University of Athens (Greece) summarized the current state of knowledge on the diagnosis and treatment of cutaneous squamouscell carcinoma (cSCC) with reference to various international guidelines [1]. The European guideline (EDF/EADO/EORTC) published in 2020 is currently under revision – the new version is expected to be published soon – and the US guidelines (NCCN) are published annually [1–4].
cSCC is more common in men and occurs in 80% of cases in the head and face area or in areas with chronic UV light exposure. The risk of disease increases significantly with age. If the presence of cSCC is suspected, inspection of the entire integument is recommended. “We should describe the lesions well, document the symptoms, and also measure the size of the lesions,” emphasized Prof. Stratigos [1]. In addition to dermoscopy, the use of other noninvasive diagnostic methods such as confocal laser microscopy and optical coherence tomography is useful, especially for differential diagnostic purposes.
The cSCC can metastasize primarily to the regional lymph nodes and form distant metastases. Therefore, early diagnosis and risk-adapted therapy are crucial. If there is a clinical suspicion of cSCC, histology should also be obtained to differentiate it from other benign or malignant neoplasia. If the clinical picture is clear for cSCC, complete resection is recommended.
Excision with histological clarification is still considered standard therapy
The goal of surgical excision is complete resection, including reprocessing of the peripheral and deep incision margins [1]. Local surgical therapy is strongly influenced by the accuracy of histologic incision margin control, Prof. Stratigos explained. For histological reporting of cSCC, a standardized reporting system should be used whenever possible with information on the following criteria: Histologic subtype (desmoplastic, acantholytic, etc.), histologic degree of differentiation, maximum tumor thickness, depth of tumor invasion (above vs. beyond subcutaneous fat), perineural invasion, lymphatic/vascular invasion, complete resection possible/not possible, minimum and maximum incision margins [1].
Sentinel lymph node biopsy: useful?
The indication for performing sentinel lymph node biopsy (SLNB) in patients with cSCC is controversial, Prof. Stratigos reported [1]. Due to lack of valid data on prognostic and therapeutic value, SLNB is not recommended as standard in the European guideline. There is no evidence that sentinel lymph node-positive patients have worse outcomes than those with negative findings, the speaker explained.
What are the most important prognostic factors?
Between the European guideline published in 2020 and the US guideline published in 2022, there is consensus that, in addition to immunosuppression, the following tumor-specific factors significantly increase the risk of metastasis and disease-specific mortality in patients with cSCC [1–4]:
- vertical tumor thickness (>6 mm)
- horizontal tumor diameter (>2 cm or >4 cm)
- Histological differentiation
- Desmoplasia
- perineural growth
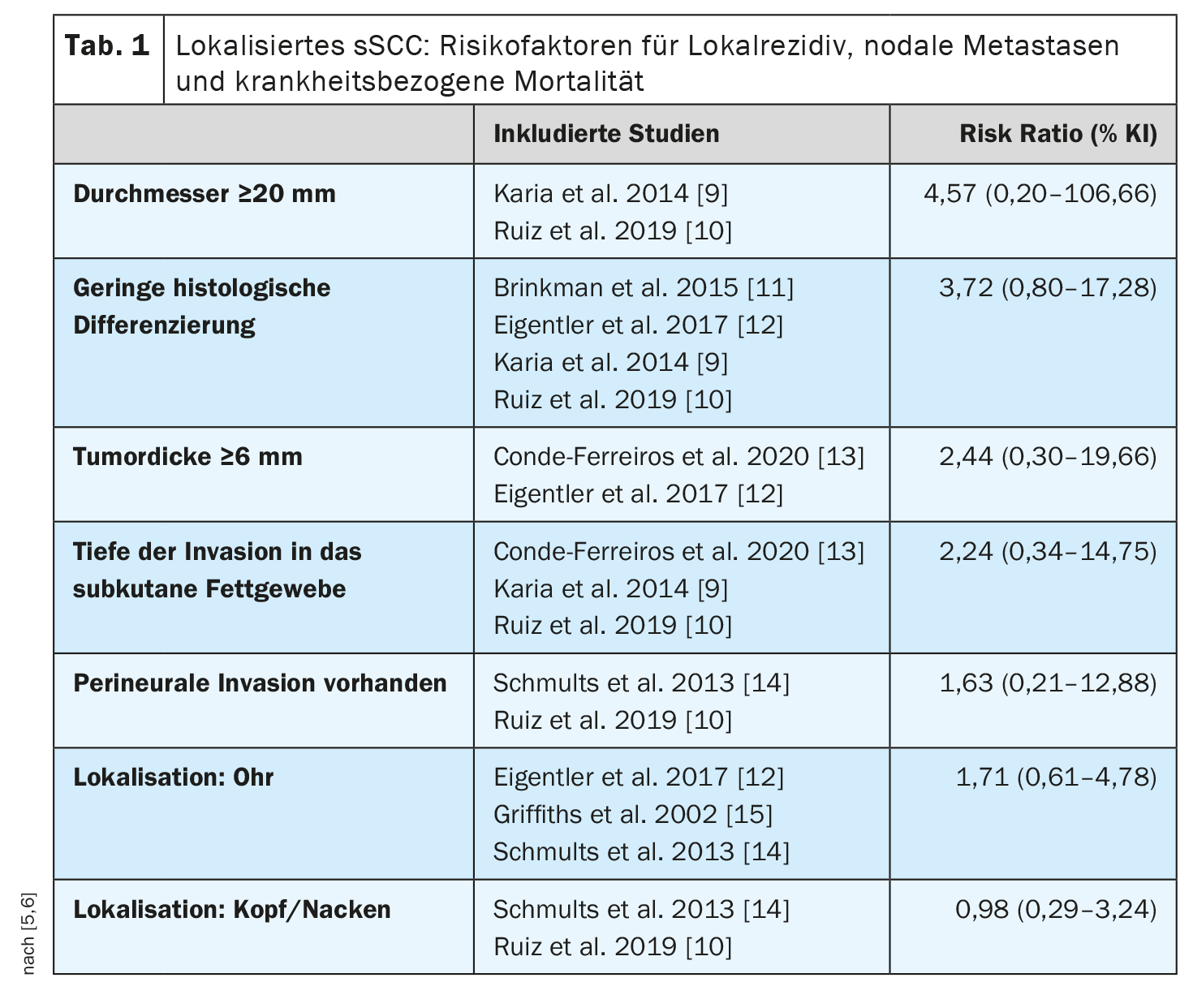
This is consistent with a meta-analysis published in 2022 that identified risk factors associated with disease-related death in patients with localized cSCC (without locoregional or distant metastases) [5,6] (Table 1). Nine studies with 5205 patients and a median follow-up of 18-81 months were included. There was a positive but nonsignificant overall association between disease-related death and tumor diameter, tumor thickness, presence of perineural invasion, depth of invasion into adipose tissue, and location. Tumor diameter ≥20 mm was associated with a 4-fold increased risk, and tumor thickness ≥6 mm was associated with more than twice the risk compared with cSCC without these features. Patients with immunosuppression were almost twice as likely to die from cSCC as immunocompetent patients (risk ratio 1.85; 95% CI: 1.32-2.61). The authors of the meta-analysis point out that these results should be considered against the background of certain methodological limitations (e.g., heterogeneity of studies).
Radiotherapy: indication recommendation
Prof. Stratigos summarized the general indications for radiotherapy as follows [1]: First, as an alternative to surgical excision in locally non-resectable tumors, inoperable patients, or difficult-to-operate tumors, or when resection is not desired. And secondly, after excision has been performed in case of positive incision margins and if reexcision is not possible. As well as in the adjuvant setting after therapeutic lymphadenectomy when cSCC was located in the head and neck with regional nodal metastases and extracapsular extension. A controversial question is whether adjuvant radiotherapy should be performed after complete excision with tumor-free resection margins. To date, no clear benefit has been demonstrated. The speaker recommends weighing this up on a case-by-case basis. Adjuvant radiotherapy may be appropriate if multiple risk factors are present.
System therapy with anti-PD-1-Ak or with chemotherapy or EGFR-i
Locoregional recurrence should be surgically removed if clinically locally possible [7]. For the treatment of local or locoregional recurrence, the indication for systemic therapy (Table 2) should be considered if no surgical or radiotherapeutic options are available. The indication and determination of system therapy should be made in a multidisciplinary tumor board, Prof. Stratigos emphasized.
In advanced cSCC or when surgical excision and radiotherapy are not possible, both the European and US guidelines suggest first-line immunotherapy with anti-PD-1-Ak (cemiplimab, pembrolizumab) [1–4]. For patients in whom checkpoint inhibitors are contraindicated, agents that block the epidermal growth factor receptor (EGFR) or various chemotherapy regimens are available [1–4].
Risk-adapted follow-up intervals
For follow-up of patients with cSCC, intervals of 6-12 months are recommended for low-risk patients over a period of 2-5 years [1]. In patients at increased risk of metastasis or with unclear palpation findings, smaller intervals should be selected in the first two years and lymph node ultrasonography should be performed each time. In locally advanced or metastatic cases, an individualized follow-up regimen is appropriate. In addition to sun protection as a general prophylactic measure, nicotinamide is advocated as a chemopreventive intervention. Nicotinamide (vitamin B3) improves, among other things, the repair of DNA damage caused by ultraviolet (UV) radiation [1]. In a randomized-controlled double-blind phase III study, nicotinamide (500 mg/2× daily) resulted in a 30% reduction in recurrent cSCC in the studied patient population (n=386) after 12 months [8].
Congress: EADO Annual Meeting
Literature:
- «Cutaneous squamous cell carcinoma guidelines», Prof. Alexander J. Stratigos, MD, EADO Annual Meeting 20–22 April 2023.
- Stratigos AJ, et al.: European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur J Cancer 2020; 128: 60–82.
- Stratigos AJ et al.: European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. Eur J Cancer 2020; 128: 83–102.
- Schmults C, et al.: National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Squamous Cell Skin Cancer 2022; Version 2.2022. www.nccn.org, (last accessed 07/14/2023).
- Dessinioti C, Stratigos AJ: Recent Advances in the Diagnosis and Management of High-Risk Cutaneous Squamous Cell Carcinoma. Cancers 2022; 14(14): 3556.
- Dessinioti C, Platsidaki E, Stratigos AJ: A Sensitivity Meta-Analysis of Disease-Specific Death in Localized Cutaneous Squamous Cell Carcinoma. Dermatology 2022; 238(6): 1026–1035.
- AWMF: Aktinische Keratose und Plattenepithelkarzinom der Haut, S3-Leitlinie 032-022OL, Registernummer 032–022OL.
- Chen AC, et al.: A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N Engl J Med 2015; 373: 1618–1626.
- Karia PS, et al.: Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol 2014; 32: 327–334.
- Ruiz ES, et al.: Surgery and Salvage Limited-Field Irradiation for Control of Cutaneous Squamous Cell Carcinoma with Microscopic Residual Disease. JAMA Dermatol 2019; 155: 1193–1195.
- Brinkman JN, et al.: The Effect of Differentiation Grade of Cutaneous Squamous Cell Carcinoma on Excision Margins, Local Recurrence, Metastasis, and Patient Survival. Ann Plast Surg 2015; 75: 323–326.
- Eigentler TK, et al.: Survival of Patients with Cutaneous Squamous Cell Carcinoma: Results of a Prospective Cohort Study. J Investig Dermatol 2017; 137: 2309–2315.
- Conde-Ferreirós A, et al.: Patterns of incidental perineural invasion and prognosis in cutaneous squamous cell carcinoma: A multicenter, retrospective cohort study. J Am Acad Dermatol 2020; 84: 1708–1712.
- Schmults CD, et al.: Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol 2013; 149: 541–547.
- Griffiths RW, Feeley K, Suvarna SK: Audit of clinical and histological prognostic factors in primary invasive squamous cell carcinoma of the skin: Assessment in a minimum 5 year follow-up study after conventional excisional surgery. Br J Plast Surg 2002; 55: 287–292.
- Yanofsky VR, Mercer SE, Phelps RG: Histopathological Variants of Cutaneous Squamous Cell Carcinoma: A Review. Journal of Skin Cancer 2011: 1–13.
DERMATOLOGIE PRAXIS 2023; 33(4): 32–33