Incretin mimetics are an attractive treatment option, particularly for the subgroup of overweight type 2 diabetics, as they not only have glucose-lowering effects, but have also demonstrated clinical benefits in terms of weight control and other parameters. In addition to several GLP-1 receptor agonists, a dual GIP/GLP-1 agonist is now also available for the indication of type 2 diabetes.
A patient-centered approach is favored in today’s diabetes management. Thanks to an evolution of treatment options, the choice of medication can be individually tailored to patient characteristics. The treatment goals have long since gone beyond good blood glucose control; other adjustments are also being made to prevent diabetes-related complications and improve quality of life. Around 90% of people with type 2 diabetes are overweight and obesity is considered one of the main risk factors for developing type 2 diabetes [1]. Studies show that a weight reduction of 5-10% improves both the HbA1c value and cardiovascular risk factors [2–4]. In view of this, lowering blood glucose and body weight are therapeutic goals that are closely linked [5]. Incretin-based drugs such as GLP-1 receptor agonists (GLP-1-RA) or dual GIP/GLP-1 agonists not only serve to control glycaemia, but also have an appetite-suppressing effect and offer other benefits (Fig. 1) .
Incretin effect through the peptide hormones GLP-1 and GIP
The so-called incretin effect is an increased insulin secretion with enteral compared to parenteral glucose intake or, in other words, the difference in the beta cell response between oral and intravenous glucose administration in a glucose tolerance test [6]. Glucagon-like peptide-(GLP)-1 is a peptide hormone which, along with glucose-dependent insulinotropic peptide (GIP), is the most important hormone for the incretin effect. GLP-1 receptor agonists (GLP-1-RA) are synthetic polypeptides that act as agonists at the GLP-1 receptor and are also known as incretin mimetics [6]. The GLP-1 RAs currently available for the treatment of type 2 diabetes have not only proven to be effective in terms of HbA1c control, but also promote weight loss [8]. In addition, large cardiovascular endpoint trials (CVOT) have shown that GLP-1-RA reduces serious cardiovascular events as well as classic antidiabetic effects and other effects [9–11]. The therapeutic use of incretin hormones initially focused on GLP-1 [12] for a long time. GIP only became the focus of research and clinical attention with the development of dual agonists such as tirzepatide, which can activate the GIP receptor as well as the GLP-1 receptor [12].
Short- and long-acting GLP-1 receptor agonists
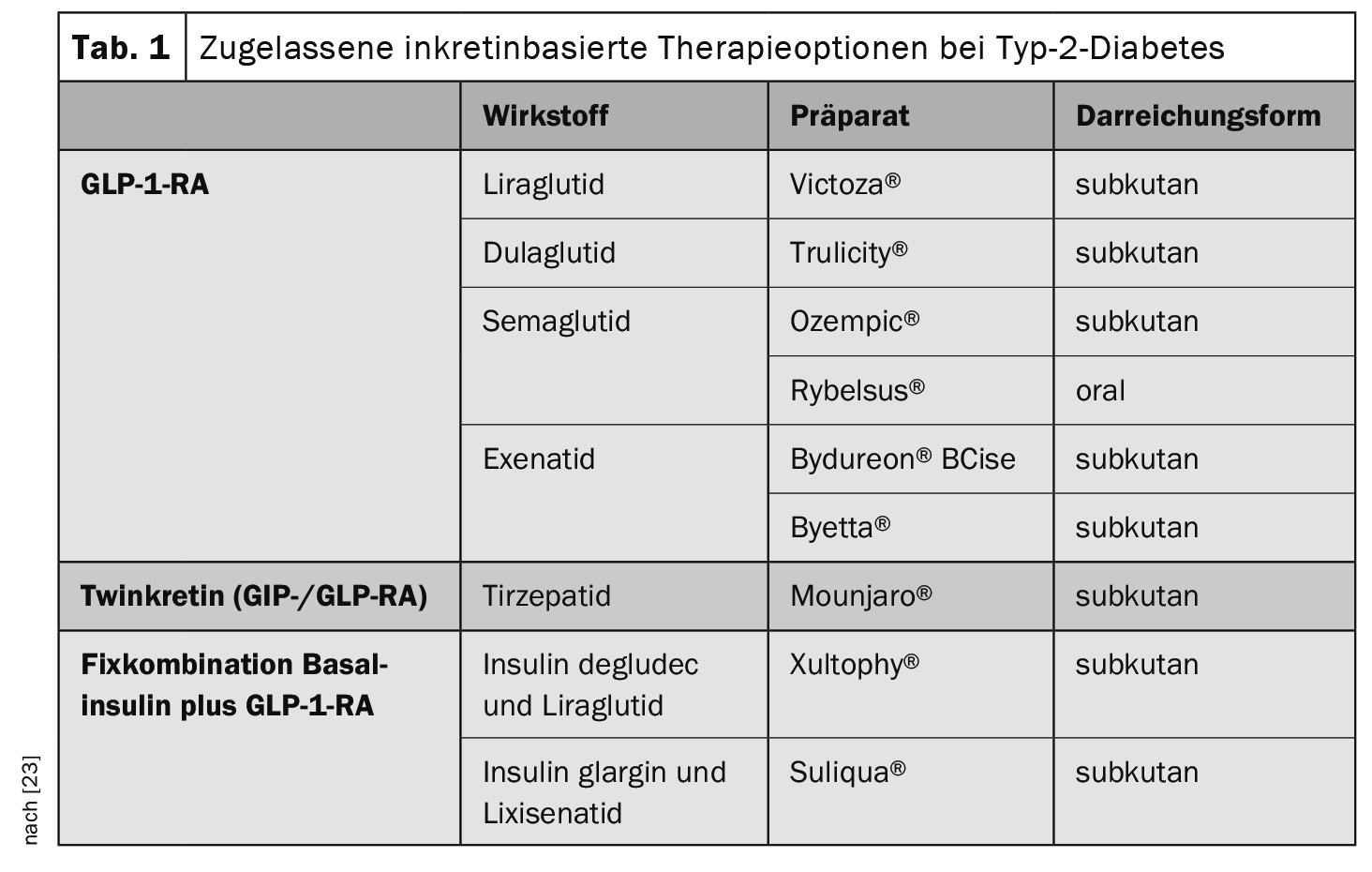
Several GLP-1 RAs are currently approved for the subcutaneous or oral treatment of type 2 diabetes (Table 1) . GLP-1 RAs can be combined with metformin and other oral antidiabetics (except DPP-4 inhibitors) and/or basal insulins. It has proven useful to distinguish between short-acting and long-acting GLP-1 RAs [13]. Short-acting GLP-1-RA are characterized by a relatively short elimination half-life and are injected at least once a day. However, there are phases within a 24-hour period during which the circulating concentrations of the active substance are low. In contrast, long-acting GLP-1 RAs are characterized by relatively small fluctuations over the course of the day (or week). The relevance of the distinction between short-acting and long-acting GLP-1-RA lies in the different influence on postprandial glucose increases: short-acting GLP-1-RA reduce these by a sustained delay in gastric emptying and long-acting GLP-1-RA by stimulation of insulin secretion and suppression of glucagon secretion [13].
Combination of GLP-1-RA with basal insulin
Fixed combinations of long-acting insulin plus GLP-1-RA or free combinations (simultaneous or consecutive) offer advantages over intensified insulin therapy with prandial and basal insulin in terms of treatment adherence, hypoglycemia rates, weight progression and insulin consumption. However, gastrointestinal side effects occurred more frequently than with intensified insulin therapy [13,16]. A recent meta-analysis showed that combinations of basal insulin with long-acting GLP-1 RAs were superior to combinations of basal insulin with short-acting GLP-1 RAs in terms of weight reduction, HbA1c reduction, lower fasting glucose levels and gastrointestinal side effects [14,15].
Dual GIP/GLP-1-RA: Twinkretin on the rise
Preclinical studies, in which the simultaneous administration of GLP-1 and GIP improved both glucose control and body weight compared to the respective monotherapy, led to an increasing focus on this therapeutic approach in type 2 diabetes [25–27]. Tirzepatide, a dual receptor agonist that combines the effects of the glucose-dependent insulinotropic peptide (GIP) and the GLP-1-RA liraglutide in a new molecule, has achieved a breakthrough in clinical application [13,14]. This Twinkretin can bind and activate both GIP receptors and GLP-1 receptors [17]. The SURPASS studies show that tirzepatide significantly improves glycemic control and greatly reduces body weight in patients with type 2 diabetes [5]. These were Phase III clinical trials on the efficacy and safety of GIP/GLP-1-RA. Tirzepatide 5, 10 and 15 mg was investigated as monotherapy and in combination with approved oral antidiabetics and/or insulin in a group of patients with type 2 diabetes: in SURPASS 1 and SURPASS 5 vs. placebo, in SURPASS 2 vs. semaglutide, in SURPASS 3 vs. insulin degludec, in SURPASS 4 vs. insulin glargine in patients with increased cardiovascular risk [18–22]. The safety data indicate that tirzepatide is generally well tolerated, has a comparable gastrointestinal side effect profile to currently available GLP-1 RAs and is associated with a low rate of hypoglycemic events** [18–22].
** except in combination with a sulfonylurea or insulin glargine
Literature:
- Hartmann B, et al: Lean diabetes in middle-aged adults: A joint analysis of the German DIVE and DPV registries. PLoS One 2017; 12: e0183235.
- Lean ME, et al: Primary care-led weight manage ment for remission of type 2 diabetes (DiRECT): an open-label, cluster randomized trial. Lancet 2018; 391: 541-551.
- Van Gaal L, Scheen A: Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care 2015; 38: 1161- 1172.
- Look AHEAD Research Group. Gregg EW, et al: Association of the magnitude of weight loss and changes in physical fit ness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomized clinical trial. Lancet Diabetes Endocrinol 2016; 4: 913- 921.
- Aberle J, et al: Tirzepatide: Tirzepatide: GIP/GLP-1 receptor agonist for the treatment of type 2 diabetes – SURPASS study program. Diabetol Metabolism 2023; 18: 461-474.
- Flexikon: Inkretin, https://flexikon.doccheck.com/de/Inkretin,(last accessed 28.02.2024)
- Gallwitz B: Clinical perspectives on the use of the GIP/GLP-1 receptor agonist tirzepatide for the treatment of type-2 diabetes and obesity. Front. Endocrinol 2022, 13:1004044. https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2022.1004044/full#B47,(last accessed 28.02.2024)
- Nauck MA, Meier JJ: Management of endocrine disease: Are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol 2019; 181: R211-R234.
- Marso SP, et al: Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
- Marso SP, et al: Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834-1844.
- Gerstein HC, et al: Dulaglutide and renal outcomes in type 2 diabetes: An exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet 2019; 394: 131-138.
- Nauck, M: Incretin agonists: From insider tip to bestseller in 30 years. Dtsch Arztebl 2023; 120(44): [6]; DOI: 10.3238/PersDia.2023.11.03.01
- Landgraf R, et al: Therapy of type 2 diabetes [Treatment of type 2 diabetes]. Diabetology 2023: 1-38.
- Landgraf R, et al: Therapy of type 2 diabetes [Treatment of type 2 diabetes]. Diabetology 2022; 18(5): 623-656.
- Huthmacher JA, Meier JJ, Nauck MA: Efficacy and safety of short- and long-acting glucagon-like peptide 1 receptor agonists on a background of basal insulin in type 2 diabetes: a meta-analysis. Diabetes Care 2020; 43: 2303-2312.
- Maiorino MI, et al: Insulin and glucagon-like peptide1 receptor agonist combination therapy in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2017; 40: 614-624.
- Coskun T, et al: LY3298 176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab 2018; 18: 3-14.
- Rosenstock J, et al: Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 di abetes (SURPASS-1): a double-blind, randomized, phase 3 trial. Lancet 2021; 398: 143-155.
- Dahl D, et al: Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022; 327: 534-545.
- Frias JP, et al: Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021; 385: 503-515.
- Ludvik B, et al: Once-weekly tirzepatide versus once daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomized, open-label, parallel-group, phase 3 trial. Lancet 2021; 398: 583-598.
- Del Prato S, et al: Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a rando mized, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021; 398: 1811-1824.
- Drug information, www.swissmedicinfo.ch,(last accessed 28.02.2024)
- Nauck MA, et al: The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes Metab 2021; 23 Suppl 3: 5-29.
- Bokvist K, et al. LY3298176, a novel long-acting GIP/GLP-1 coagonist, shows enhanced activity on weight loss and energy utilization whilst maintaining its efficacy for glycaemic control. Diabetologia 2017; 60 (Suppl. 1): S399.
- Finan B, et al: Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med 2013; 5: 209ra151.
- Tschöp MH, et al: Unimolecular Polypharmacy for Treatment of Diabetes and Obesity. Cell Metab 2016; 24: 51-62.
FAMILY PHYSICIAN PRACTICE 2024; 19(3): 24-25