In malignant melanoma, some new findings on prognostic factors and risks have been published recently. The changed guidelines bring some innovations in everyday life and aim at a most accurate subdivision between high-risk and low-risk melanomas. The greatest changes occur primarily in thin melanomas between 0.75 and 1 mm, for which additional new criteria have been created. However, the future of melanoma diagnostics will undoubtedly involve work with melanoma markers and mutation analysis, which are already routinely used today for the treatment of advanced metastatic melanoma.
The incidence rate of melanoma is very high, especially in the younger population. In addition, metastases occur relatively early, for which no cure has yet been found. Malignant melanoma is the most common fatal tumor in young adults, making it the third leading cause of death after accidents and suicide. In the future, therefore, the focus should not only be on the treatment of melanoma, but also in particular on prevention and the identification of risk groups for whom regular examination and screening is worthwhile.
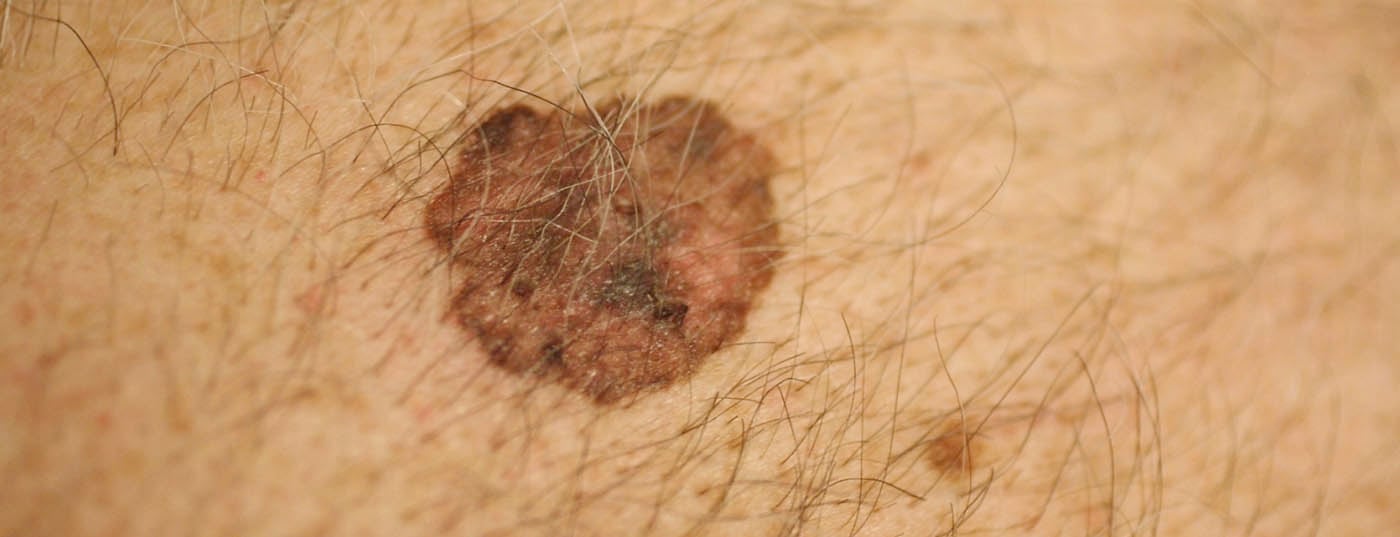
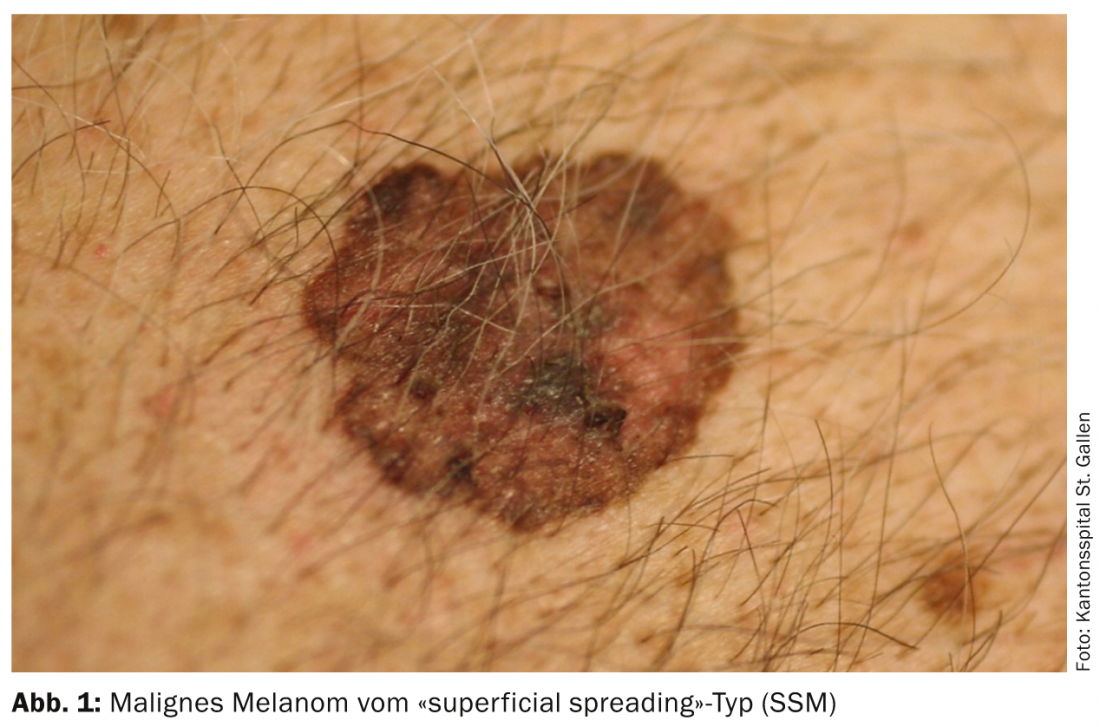
The huge increase in melanomas has largely to do with changes in leisure time behavior. Thus, young skin is exposed to the sun earlier and more intensively, especially during bathing vacations, in the beach band, solarium, etc.. In particular, the so-called “superficial spreading melanoma” (SSM) develop. (Fig.1) and nodular melanomas on body sites that do not even have much cumulative exposure to the sun, but experience switch-on and switch-off exposure to the sun. In addition, the higher mixing of ethnicities in southern latitudes is also significant, as Caucasians with a residence near the equator have a significantly higher incidence of melanoma, especially those of central and northern European descent. The incidence rate in Switzerland is over 220 persons per 1 million population per year, the highest rate in Europe.
Risk groups
Previous prevention campaigns focused more on high-risk skin, i.e. skin types I and II, which suffer more sunburn without sufficient sun protection. However, the true risk patients, which are listed in Table 1 , have been rather neglected so far. Therefore, prevention measures regarding sun protection should be applied to everyone, since it is not possible to distinguish between low-risk and high-risk individuals in the general population.

In particular, the number of dysplastic nevus cell nevi, and this especially in the gluteal region, indicates an up to ninefold increased risk of melanoma compared to the normal population. The other risk groups listed in Table 1 also have a 2.3-18-fold increased risk of melanoma. It is therefore important to also include precisely those patients (group 5 of the Tab. 1), who have already been treated for another tumor should also be informed about the risk of melanoma and examined regularly. Also, it is important to point out risk already in children and certainly perform a total body examination incl. the gluteal and genital region.
Men have an additional risk. They have a worse prognosis and higher mortality regardless of melanoma thickness. We find further risk factors on the side of genetics; especially in familial melanomas, inherited mutations of two genes with a critical role in the cell cycle, namely CDKM2A and CDK4, are postulated. However, additional trigger factors such as sun exposure and other environmental factors also play a role in this case. In addition, many other gene loci are involved, which differ depending on the type of melanoma, whether it is a lentigo-maligna melanoma or a uveal melanoma, for example.
Lentigo-maligna melanoma, moreover, is more associated with advanced age and cumulative total sun exposure. It just appears on the face, upper back and upper extremities.
Aspirin use for more than five years was found to be a weak protective factor, as well as nicotine use for lentigo-maligna melanoma. The use of TNF-α inhibitors (biologics) in the treatment of inflammatory bowel disease results in an increased incidence of melanoma compared with TNF-α free patients. These patients have a higher risk of developing melanoma than, for example, family members of melanoma patients who have no additional risk factors.
Classification of melanoma and changes
To date, melanoma thickness is the most important prognostic factor and leads to therapeutic decisions. Not much has changed in this primarily surgical approach with regard to post-excision. The rule still applies: below 1 mm thickness according to Breslow, 1 cm is recut; above 2 mm thickness, 2 cm. For in situ melanoma, a safety distance of 0.5 cm is chosen.
Newly, the assessment of the mitotic index is crucial for further investigation such as sentinel lymph node removal. Newly, for melanoma between 0.75 mm and 1 mm thick, the mitotic rate in one square millimeter is also looked at. If this is greater than 1, a sentinel lymph node examination should be performed, as with a thicker melanoma greater than 1 mm. However, this has no effect on overall survival and freedom from recurrence, but is used to detect micrometastases and thus also to indicate lymphadenectomy. In addition, there is a possibility that in future studies these high-risk patients with micrometastases may also be treated prospectively and/or adjuvantly according to melanoma mutation analysis.
However, it is important that the re-excision takes place within one month, because this also has an impact on the overall survival of the patient. The primary excision should be performed narrowly, and the post-excision should be performed together with the sentinel lymph node at most, but within one month.
Here, the new TNM classification (Tables 2 and 3) takes into account the mitotic rate, which should be assessed for melanomas less than 1 mm thick.

An additional important factor is ulceration, which may be indicative of a different tumor defense immunotype and may warrant adjuvant interferon-α therapy in tumor stage IIb, i.e., above 2 mm Breslow with ulceration.

Who should care for the high-risk patients?
Today, it is already ensured that a three-year dermatological specialist training is crucial for melanoma detection. In addition, high-risk patients should also be examined at least annually with the inclusion of dermatoscopic examination. Full body photographs and comparison photographs should be taken or even digital storage media such as Molemax should be used.
Can screening also be done at the primary care physician’s office?
Undoubtedly, the risk population is too large to be examined by a specialist as a whole, which would actually be recommended. In fact, it is more important that those at risk, especially middle-aged men between 40 and 60 years of age, go for the checkup in the first place. This should be the primary goal of any melanoma campaign. In addition, however, more training for primary care physicians regarding dermoscopy standards should be developed and offered. A special skills diploma would be useful, even for non-dermatologists.
Further diagnostics in the presence of melanoma
According to the ADO S3 guideline, initial spread diagnosis is useful for melanomas up to stage IIb, i.e. melanomas up to 4 mm with or without ulceration without lymph node or distant metastases are treated according to staging incl. Sentinel lymph node biopsy scanned annually and S-100 protein tested annually for a period of five years. Clinical examinations are performed every six months. In addition, stage Ib patients, i.e., melanomas less than 1 mm but with ulceration and a higher mitotic rate of ≥1/mm2, are also candidates for annual ultrasonography and serum S-100 measurement. MRI, CT, chest X-ray, abdominal ultrasonography, and skeletal scintigraphy are not recommended in the follow-up of these melanomas up to 4 mm thick.
PET-CT examination: From melanomas of 4 mm thickness and in selected situations as well as in very young patients, a PET-CT examination may also be recommended as primary diagnostics before sentinel lymph node dissection or removal is performed. The usefulness of an additional MRI of the head is questionable if the PET-CT is negative. PET-CT, on the other hand, consistently finds solitary metastases that can be addressed curatively (e.g., recurrence in scar, lymph node, solitary soft tissue metastasis between primary tumor and lymph node station).
sentinel lymph node: For any melanoma greater than 1 mm in diameter (and in special situations, 0.75 mm, Table 4) , finding and selectively removing the sentinel lymph node is reasonable (should no enhancements be found on PET-CT for a melanoma greater than 4 mm Breslow).

Workup of sentinel lymph nodes is recommended in the S3 guideline according to national or international protocols. It makes sense here to work up with at least eight tissue sections and define whether micrometastases (melanoma cell conglomerates, indicated in tenths of a millimeter) or macrometastases are present. Additional factors that need to be answered are the depth of penetration of melanoma cells into the lymph node parenchyma, infiltration of the lymph node capsule, its breakthrough, and lymphangiosis. The significance of the various indications regarding tumor burden are still unclear prognostically, but should be indicated histopathologically.
Recommendations for metastasis from stage IIc (patients at high risk of recurrence): These patients should receive MRI of the head, PET-CT of the whole body, lymph node ultrasonography, tumor marker S-100 in serum, and tumor marker LDH. If locoregional metastasis is detected, abdominal ultrasonography should be performed, as well as a chest x-ray. Patients with proven micro- or macrometastases should be presented to the interdisciplinary tumor board. The consensus is that elective lymphadenectomy is not primary unless macrometastases are found in the sentinel lymph node or enhancement on PET-CT. Prophylactic lymphadenectomy in the presence of micrometastases must be discussed on a case-by-case basis. At this moment, active information of the patient and detailed discussion/support is also necessary. Furthermore, it is decided whether e.g. adjuvant therapies are useful or not.
Adjuvant radiotherapy is recommended after lymphadenectomy if three lymph nodes are affected, capsular rupture has occurred, or a lymph node metastasis greater than 3 cm is present. In the case of any metastases, it is also useful to have the specimens analyzed at a pathology institute, which can perform molecular genetic studies. In particular, BRAF mutation analysis is recommended, if negative also NRAS mutation analysis and in mucosal melanomas the examination regarding c-Kit. This is of great importance nowadays, not only for currently ongoing adjuvant studies, but also for the subsequent treatment of any metastases, against which targeted drugs can be used in the case of this mutation.
Mark David Anliker, MD
Literature:
- Nikolaou V, Stratigos AJ: Emerging trends in the epidemiology of melanoma. Br J Dermatol 2014; 170: 11-19.
- Fong ZV, Tanabe KK: Comparison of melanoma guidelines in the U.S.A., Canada, Europe, Australia and New Zealand: a critical appraisal and comprehensive review. Br J Dermatol 2014; 170: 20-30.
- Vredenborg A, et al: Acquired melanocytic nevi in childhood and familial melanoma. JAMA Dermatol 2014; 150: 35-40.
- Oncology Guideline Program, S3 Guideline, Melanoma, Version 1.0, January 2013.
CONCLUSION FOR PRACTICE
- The new TNM classification distinguishes not only according to the thickness of the melanoma, but also according to the mitotic rate, which strongly influences the staging procedure and better defines the risk of metastasis even in thin melanomas.
- Every physician is challenged to get at-risk patients into a regular checkup and to point out preventive measures as early as childhood.
- In thin melanomas, less imaging is performed for primary diagnosis, but sentinel lymph node extirpation is recommended for melanomas as thin as 0.75 mm when a higher mitotic rate is present, which may become significant with respect to adjuvant therapy and future therapeutic approaches involving, for example, BRAF, NRAS inhibition, and inhibition of newer molecular targets.
- Standards should be introduced for general practitioners and dermatologists, possibly including courses with skill certificates for dermoscopy and melanoma detection.
A RETENIR
- La nouvelle classification TNM fait la différence non seulement en fonction de l’épaisseur du mélanome, mais également en fonction du taux de mitoses qui a des répercussions importantes sur la classification et également définit mieux le risque de métastase dans le mélanome de faible épaisseur.
- Tout médecin se doit de proposer aux patients à risque des contrôles réguliers et de les informer des mesures de prévention dès l’enfance.
- L’imagerie est peu utilisée pour le diagnostic initial dans les mélanomes de faible épaisseur, cependant l’exérèse des ganglions sentinelles est recommandée dès l’épaisseur de 0,75 mm des mélanomes, en présence d’un taux de mitose élevé, ce qui peut être pertinent en ce qui concerne le traitement adjuvant et les futures approches thérapeutiques englobant par exemple l’inhibition de BRAF, NRAS et l’inhibition de nouvelles cibles thérapeutiques.
- Des normes doivent être définies pour les médecins généralistes et les dermatologues, au mieux également des enseignements avec attestation de capacité pour la dermatoscopie et la reconnaissance des mélanomes.
DERMATOLOGIE PRAXIS 2014; 24(3): 6-10