Over the last two decades, the evidence has become increasingly clear that even relatively mild hyponatremia without obvious or severe symptoms is clinically relevant. It leads to neurocognitive impairments, gait instability and an increased risk of falling. Hyponatremia also increases the risk of osteoporosis through increased osteoclast activation, which, together with the higher risk of falling, leads to an increased fracture rate. Taken together, there are good arguments for attaching importance to sodium levels in the outpatient setting as well.
Over the last two decades, the evidence has become increasingly clear that even relatively mild hyponatremia without obvious or severe symptoms is clinically relevant. Hyponatremia leads to – sometimes subtle – neurocognitive impairments, gait instability and an increased risk of falling [1,2]. Patients with hyponatremia performed worse in geriatric assessments and correcting hyponatremia improved performance [3]. Hyponatremia also increases the risk of osteoporosis through increased osteoclast activation, which, together with the higher risk of falling, leads to an increased fracture rate. Even an association between hyponatremia and long-term mortality has been described – also in the outpatient setting – although the direction of causality has of course not been proven with certainty. Taken together, there are good arguments for attaching importance to sodium levels in the outpatient setting as well, and with the appropriate knowledge, mild to moderate hyponatremia can also be managed well by the general practitioner – apart from more specialized cases.
When and on whom should the sodium be measured?
Determining the sodium level in an outpatient setting is useful in the case of symptoms that could be caused by hyponatremia. Accordingly, a measurement of the sodium level is always part of the clarification of a clouding of consciousness, delirium or a first seizure. These illnesses are of course usually treated as inpatients or in hospital. However, as mentioned at the beginning, the symptoms can be discrete and non-specific, especially in cases of mild hyponatremia. A sodium determination also makes sense in cases of unsteady gait, falls, cognitive deficits or concentration disorders – especially in older patients. On the other hand, there are some risk factors for hyponatremia, such as certain medications, tumor diseases, heart failure and liver cirrhosis, in the presence of which occasional sodium checks are useful, even if no symptoms are present.
Causes and clarification of hyponatremia
If hyponatremia is detected, its cause should be investigated, as the therapy differs considerably depending on this. Nevertheless, as a pragmatic first step before further clarification, it is worthwhile to discontinue medications that can trigger hyponatremia and that are not necessarily indicated. These primarily include thiazide diuretics and certain psychotropic drugs. If the hyponatremia does not improve, or if the possible triggers are urgently indicated medications, there is no getting around some pathophysiological considerations and further clarification.
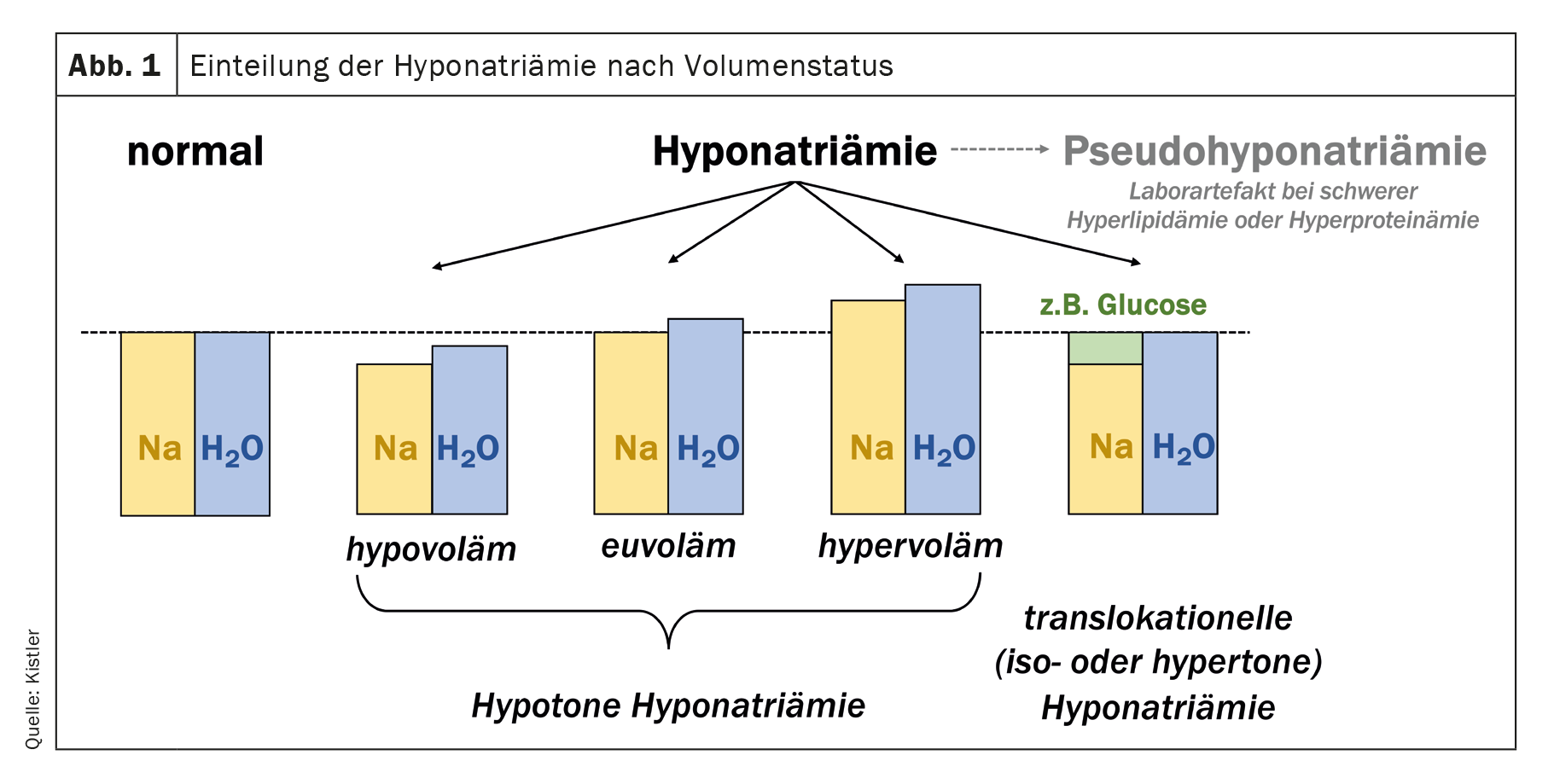
Hyponatremia does not mean a sodium deficiency – accordingly, affected patients should not be told that they have “too little salt in their bodies”! This often leads to misconceptions and wrong therapeutic approaches. Total body sodium can be reduced in hyponatremia, while total body water is less severely reduced (hypovolemic hyponatremia), but it can also be normal with increased total body water (euvolemic hyponatremia) or even increased with even more increased total body water (hypervolemic hyponatremia) (Fig. 1) . Special cases are translocational hyponatremia and pseudohyponatremia. The former occurs mainly in hyperglycemia, where water flowing out of the cells counteracts the glucose-induced extracellular hypertonicity. As a parenthetical note, alcohol and urea are ineffective osmolytes that pass freely through the cell membrane and do not lead to translocational hyponatremia. Hyponatremia with simultaneous alcohol intoxication with high serum osmolality is therefore usually a true (hypotonic) hyponatremia: the plasma osmolality is increased, but the tonicity (also called effective osmolality) is decreased. Pseudohyponatremia is a rare laboratory artifact in severe hyperlipidemia or hyperproteinemia. It can be detected by determining the lipid and protein concentration or by determining the sodium in the blood gas analysis, which is not affected by this artifact. However, pseudohyponatremia is rare and only needs to be looked for if there are corresponding indications (e.g. multiple myeloma, pancreatitis).
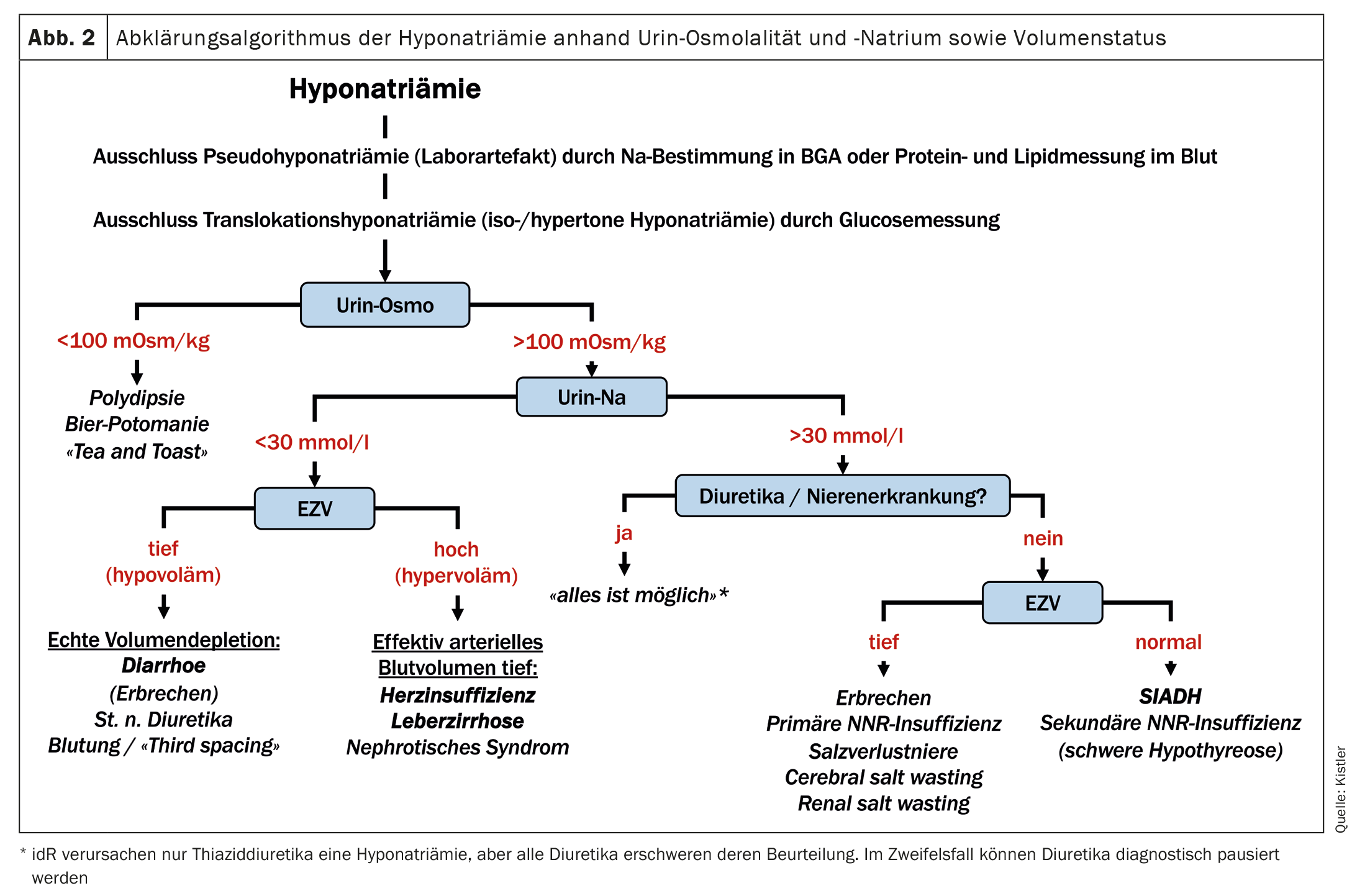
True (hypotonic) hyponatremia is therefore primarily a disorder of the water balance rather than the sodium balance. The crucial question now is why the kidneys retain water. The central hormone in the regulation of extracellular tonicity is ADH. ADH secretion is regulated on the one hand by plasma tonicity and on the other hand by the effective arterial blood volume (EABV) via baroreceptors. The volume stimulus has priority: with reduced EABV, ADH is released despite low plasma tonicity. In this situation, in addition to ADH, the renin-angiotensin-aldosterone system (RAAS) is also activated, which leads to sodium retention. Urine osmolality and urine sodium (both in spot urine, i.e. in a urine sample taken at any time) are used for the clinical assessment of these hormone systems. The former is <100 mOsm/kg with maximally suppressed ADH – conversely, a urine osmolality of >100 mOsm/kg indicates a non-maximally suppressed ADH, either due to a volume stimulus or due to inadequate ADH secretion. A urine sodium concentration of <30 mmol/l indicates an activated RAAS. However, caution is advised here with diuretics or tubular kidney damage. In this case, the urine sodium is generally 30 mmol/l even with a volume deficiency >and therefore cannot be assessed. Hypotonic hyponatremia can now essentially be caused by the following mechanisms, which can be differentiated on the basis of the aforementioned laboratory values in the urine and the volemia (Fig. 2) [4]:
- A: Polydipsia: massively increased water intake that exceeds the renal elimination capacity. This is rather rare, as a healthy person can drink over ten liters of water a day without developing hyponatremia. However, if a large amount of water is drunk over a short period of time, this mechanism can lead to hyponatremia.
- B: Increased water intake with simultaneously reduced intake of osmolytes due to malnutrition. In this situation, even with maximally diluted urine, the osmolytes to be excreted are not sufficient to excrete enough water. Classic examples are beer potomania (calorie intake in the form of alcohol, which does not generate renally excreted osmolytes during metabolism) and the “tea and toast” syndrome, which occurs mainly in malnourished elderly people who consume very little protein and yet drink a relatively large amount.
- C: Reduced EABV, which stimulates the release of ADH. This can be the case with genuine hypovolemia (blood loss, diarrhea, vomiting, etc.) or also with maldistributed volume in the context of heart failure, liver cirrhosis or, rarely, nephrotic syndrome. This leads to hypervolemia and edema due to venous congestion and/or reduced plasma oncotic pressure, but at the same time to reduced arterial perfusion.
- D: Syndrome of inadequate ADH secretion (SIADH) or, more recently, syndrome of inadequate antidiuresis (SIAD). SIADH is a syndrome, not a diagnosis, and can have a variety of causes that usually fall into one of the following categories: Malignancies, lung disease, CNS disorders, medications, or acute stimuli such as nausea, pain, anesthesia. If no drug or other clinically proven cause is found and the SIADH persists, cerebral imaging and computed tomography of the thorax should be performed after exclusion of NNR insufficiency, followed by abdominal imaging to look for the causes mentioned. Constitutive activation of the ADH receptor (V2 receptor) is another possible cause (hence the newer name SIAD instead of SIADH), but is extremely rare and hardly plays a role in everyday life.
- E: Severe renal insufficiency with renal water retention. According to clinical experience, however, renal insufficiency is very rarely the sole cause of hyponatremia, as salt and water are usually retained at the same time.
The scheme shown in Figure 2 can help to identify the cause of hyponatremia based on these pathophysiological mechanisms using the parameters described above. In addition to these laboratory parameters, the medical history (fluid loss in the form of vomiting/diarrhea? Cardiac, hepatic, renal pre-existing conditions?) and the clinical examination (volume status?) are important. For those who are particularly interested, a few additions and special cases are also mentioned here:
Hypervolemia is usually easy to recognize clinically. The distinction between euvolemia and hypovolemia is more difficult. If the urine sodium cannot be utilized due to diuretic use, a determination of uric acid can sometimes be helpful: This is usually high normal or elevated in the case of reduced EABV and low normal or low in the case of SIADH. The orthostasis reaction after one minute is suitable for the clinical assessment of the volume status: a drop in blood pressure of >20 mmHg and/or an increase in pulse rate of >30% indicates hypovolemia.
An often cited hypothyroidism very rarely – if ever – leads to hyponatremia. On the other hand, adrenal insufficiency can lead to hyponatremia. In primary (peripheral) NNR insufficiency, hypovolemia occurs due to renal salt loss – however, relevant hyponatremia is rather rare here. In secondary (central) NNR insufficiency, inadequate ADH secretion occurs via hypothalamic-pituitary feedback. Accordingly, secondary NNR insufficiency can be difficult to distinguish from SIADH and the search for NNR insufficiency is therefore part of the clarification of SIADH.
In the case of vomiting, hypovolemia leads to ADH release. At the same time, the RAAS is activated, so that one would actually expect a low urine sodium. However, this is often >30 mmol/l due to bicarbonaturia (bicarbonate is excreted with sodium due to electroneutrality).
Treatment of hyponatremia in the outpatient setting
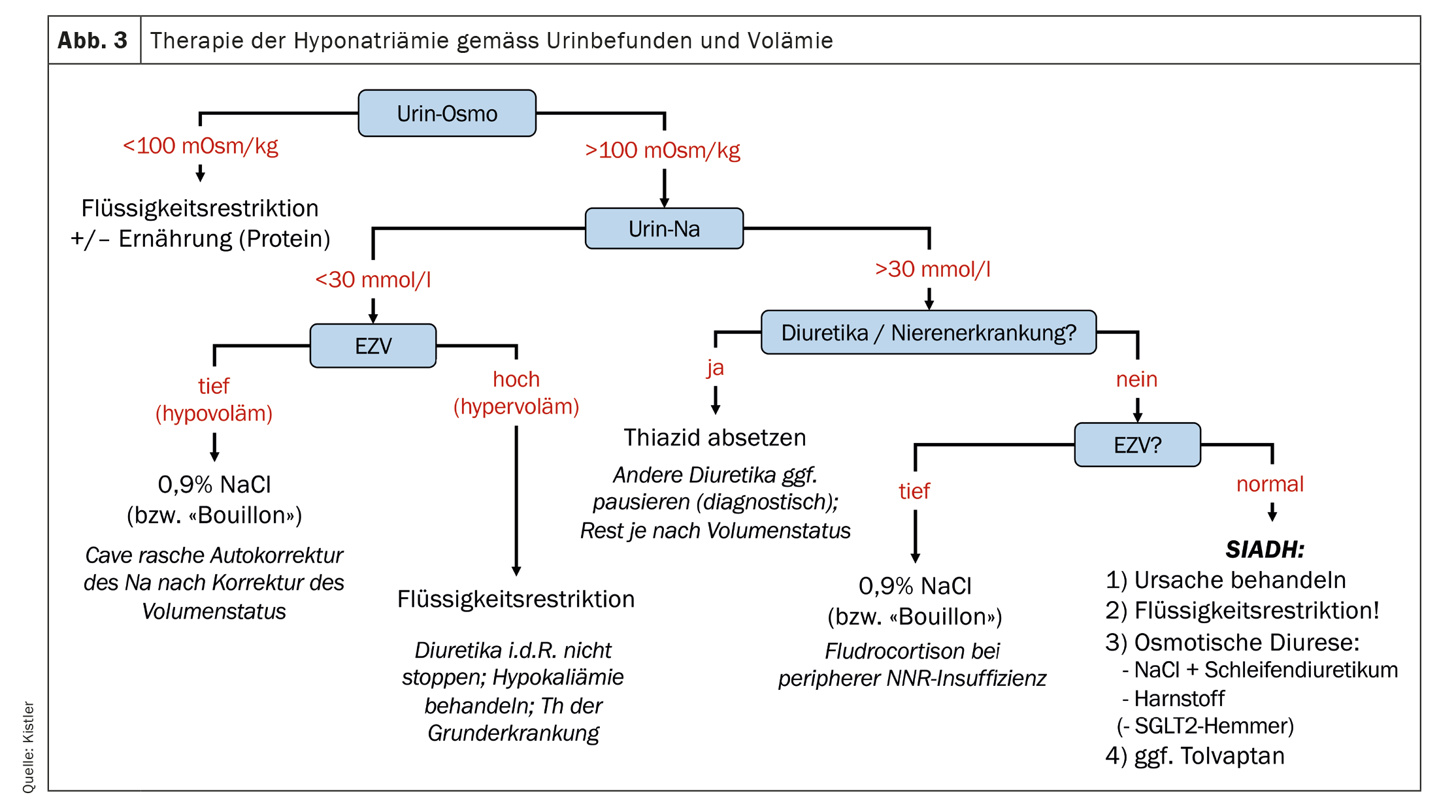
In cases of severe hyponatremia (<125 mmol/l) with severe symptoms (seizure/coma/cardiorespiratory instability), immediate therapy with 3% NaCl – preferably administered as a bolus (100-150 ml) [4,5] – under close monitoring is indicated, regardless of the cause. This therapy must take place in a hospital setting. In all other cases, the therapy depends on the result of the cause clarification described above and can often be carried out in an outpatient setting.
In the case of beer potomania or “tea and toast” syndrome, a better and more protein-rich diet should be sought in addition to limiting the amount of beer consumed. In the case of hypovolemic hyponatremia, any causes should be eliminated (e.g. discontinuation of diuretics; treatment of diarrhea, etc.) and the volume status corrected. The latter can be done by 0.9% NaCl infusion or, in an outpatient setting, by oral rehydration solutions containing salt or bouillon. Caution: If the hypovolemia is corrected quickly, the ADH stimulus is removed and rapid autocorrection may occur. Therefore, NaCl infusion in the outpatient setting without subsequent monitoring is not recommended. In the case of hypervolemic hyponatremia, fluid restriction is advisable or necessary. As a rule, any diuretic therapy should not be discontinued, as otherwise the hypervolemia will worsen. However, any salt restriction (often prescribed for heart failure) can be temporarily eased. Of course, the treatment of the underlying cardiac, hepatic or renal disease is crucial.
The treatment of SIADH is often the most challenging. As a first step, potentially triggering medication should be discontinued if possible. At the same time or as a second step, fluid restriction is recommended [6]. As a pragmatic approach, we recommend initially restricting fluids to <1l täglich, die im Verlauf angepasst werden kann (gelockert oder intensiviert). Alternativ kann vorgängig abgeschätzt werden, wie erfolgversprechend eine Flüssigkeitsrestriktion ist: Eine Urin-Osmolalität>500 mOsm/l, a urine sodium >130 mmol/l or a sum of urine sodium and urine potassium that is greater than the blood sodium have been identified as indications that very strict fluid restriction (<0.5 l/day) is necessary or that other measures in addition to fluid restriction are necessary to correct the hyponatremia [7]. In these cases, the next strategy that can be tried is to increase the excretion of osmolytes via the urine. This can also increase the excretion of water despite inadequately high urine osmolality. This can be achieved either by administering table salt in combination with a loop diuretic [8] or by administering urea (15-60 g daily) [9,10]. The administration of SGLT2 inhibitors that induce glucosuria is an elegant newer option, but it is off label and should be investigated in larger studies [11]. Finally, the ADH (V2) receptor antagonist tolvaptan has been available for several years. However, this effective therapy for SIADH is reserved for individual cases due to its extremely high price.
Figure 3 shows how the determination of urine osmolality and urine sodium and the diagnostic scheme (Fig. 2) can help in choosing the correct treatment for hyponatremia.
Conclusion
Hyponatremia is considered the most common electrolyte disorder, is prognostically relevant and is also frequently encountered in the outpatient setting. It is and remains complex – and for an adequate therapy we usually cannot avoid some further clarifications and also some basic pathophysiological considerations. I hope that this article has given you a better understanding of hyponatremia and also provided you with a few “recipes” in the form of the diagrams.
Take-Home-Messages
- Even mild hyponatremia without immediately obvious symptoms can have relevant clinical consequences – hyponatremia should therefore be specifically sought in certain situations.
- Hyponatremia does not mean a lack of sodium or salt – more often it is an excess of water.
- If the cause of hyponatremia is not immediately apparent, the urine osmolality and the urine sodium concentration in the spot urine should be determined in addition to the clinical assessment of the volume status.
- The causal therapy of hyponatremia differs greatly depending on its cause. Some pathophysiological considerations and further clarifications are therefore worthwhile.
Literature:
- Rondon-Berrios H, Berl T: Mild Chronic Hyponatremia in the Ambulatory Setting: Significance and Management. Clin J Am Soc Nephrol 2015; 10(12): 2268–2278.
- Renneboog B, Musch W, Vandemergel X, et al.: Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 2006; 119(1): 71.e1–8.
- Brinkkoetter PT, Grundmann F, Ghassabeh PJ, et al.: Impact of Resolution of Hyponatremia on Neurocognitive and Motor Performance in Geriatric Patients. Sci Rep 2019; 9(1): 12526.
- Spasovski G, Vanholder R, Allolio B, et al.: Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant 2014; 29 Suppl 2: i1–i39.
- Baek SH, Jo YH, Ahn S, et al.: Risk of Overcorrection in Rapid Intermittent Bolus vs Slow Continuous Infusion Therapies of Hypertonic Saline for Patients With Symptomatic Hyponatremia: The SALSA Randomized Clinical Trial. JAMA Intern Med 2021; 181(1): 81–92.
- Garrahy A, Galloway I, Hannon AM, et al.: Fluid Restriction Therapy for Chronic SIAD; Results of a Prospective Randomized Controlled Trial. J Clin Endocrinol Metab 2020; 105(12).
- Winzeler B, Lengsfeld S, Nigro N, et al.: Predictors of nonresponse to fluid restriction in hyponatraemia due to the syndrome of inappropriate antidiuresis. J Intern Med 2016; 280(6): 609–617.
- Krisanapan P, Vongsanim S, Pin-On P, et al.: Efficacy of Furosemide, Oral Sodium Chloride, and Fluid Restriction for Treatment of Syndrome of Inappropriate Antidiuresis (SIAD): An Open-label Randomized Controlled Study (The EFFUSE-FLUID Trial). Am J Kidney Dis 2020; 76(2): 203–212.
- Lockett J, Berkman KE, Dimeski G, et al.: Urea treatment in fluid restriction-refractory hyponatraemia. Clin Endocrinol (Oxf) 2019; 90(4): 630–636.
- Perelló-Camacho E, Pomares-Gómez FJ, López-Penabad L, et al.: Clinical efficacy of urea treatment in syndrome of inappropriate antidiuretic hormone secretion. Sci Rep 2022; 12(1): 10266.
- Refardt J, Imber C, Nobbenhuis R, et al.: Treatment Effect of the SGLT2 Inhibitor Empagliflozin on Chronic Syndrome of Inappropriate Antidiuresis: Results of a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J Am Soc Nephrol 2023; 34(2): 322–332.
InFo ONKOLOGIE & HÄMATOLOGIE 2024; 12(1): 14–18