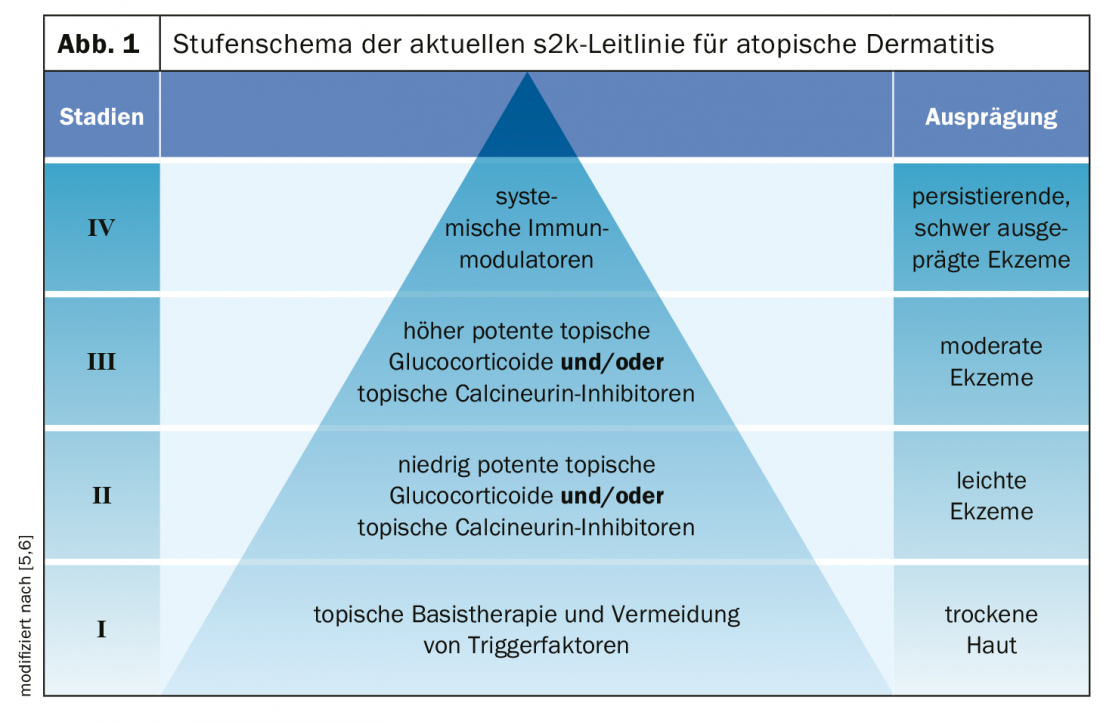
Dupilumab, a highly effective biologic, has recently become available as a second-line therapy for moderate to severe atopic dermatitis. Other targeted therapies are being explored in clinical trials. From the “small molecule” field, baricitinib is a promising JAK1 and JAK2 inhibitor that showed great therapeutic potential.
Thus, another milestone in the field of system therapy of this inflammatory dermatosis could be reached in the near future. “The pathogenesis of atopic dermatitis is extremely complex and associated with many genetic factors,” explains Julia-Tatjana Maul, MD, head of the psoriasis consultation and senior physician at the University Hospital Zurich [1]. The IL4/IL13 inhibitor dupilumab (Dupixent®) has revolutionized the treatment options for moderate and severe atopic dermatitis and the corresponding clinical studies have led to a significant gain in knowledge regarding the pathogenetic processes underlying this inflammatory dermatosis. Further targeted therapy options, which could also be used in the context of personalized treatment approaches and for which specific efficacy for certain subpopulations of patients with atopic dermatitis is indicated, are currently being explored. Among them are also some low-molecular substances.
JAK inhibitor has cleared Phase III hurdle
The mechanism of action of JAK inhibitors is to use “small molecules” to achieve blockade of Janus kinases (JAK), which cytokine receptors rely on to exert their inflammatory mediator effects via an intracellular signaling cascade inside cells [2]. Baricitinib is an oral selective JAK1 and JAK2 inhibitor that has recently met efficacy and safety endpoints. This small molecule active substance was tested in two independent multicenter randomized placebo-controlled phase III studies in a total of 1239 adult patients with moderate to severe atopic dermatitis [3].
Study participants were randomized to the baricitinib 1 mg, 2 mg, or 4 mg treatment arms. After only 1 week of treatment, the 2 mg and 4 mg conditions showed significant improvements in both itch symptoms and sleep quality, as well as quality of life. At follow-up after 16 weeks, a significantly higher proportion of subjects achieved an IGA* score of 0 or 1 (lesion-free or nearly lesion-free) in the baricitinib 4 mg and 2 mg conditions compared with placebo. In addition to a solid efficacy profile, safety data also proved favorable, with mainly mild side effects such as nasopharyngitis and headache [3].
* IGA = Validated Investigator’s Global Assessment
Further active ingredients are in the pipeline
The monoclonal antibodies tralokinumab and lebrikizumab, which belong to the group of IL13 inhibitors, have also shown a good efficacy-safety profile so far.
Tralokinumab prevents binding to IL13R-α1 and to IL13R-α2. In all three phase III trials, the primary endpoints were met by achieving significant clinical improvement defined as IGA 0/1 (lesion-free or nearly lesion-free) and EASI75 at week 16 [7]. In ECZTRA 1 and 2, 16% and 22%, respectively, of patients receiving tralokinumab 300 mg subcutaneously every two weeks met the primary endpoints, compared with only 7% and 11%, respectively, in the placebo comparison group. A total of 1596 adult patients with moderate to severe atopic dermatitis were included in the subject population of the ECZTRA 1 and 2 randomized-controlled trials. ECZTRA 3 was a double-blind, randomized study comparing efficacy and safety of tralokinumab in combination with topical steroids versus placebo. The primary endpoints were also met in this substudy. The safety profile of tralokinumab proved favorable, with headache and upper respiratory tract infections as the most common adverse events in Phase II-b studies.
Lebrikizumab also binds selectively to IL13. Unlike tralokinumab, lebrikizumab prevents the binding of IL13R-α1 and IL4R-α-heterodimer receptor signaling complex, but not the binding of IL13 to the “decoy receptor IL13R-α2,” which can take up excess IL13, the speaker explained [1]. “Whether and how this affects therapy is being shown in ongoing Phase III trials,” she said. In the phase IIb dose-finding study, a total of 280 patients with moderate-to-severe atopic dermatitis were randomized to the following lebrikizumab treatment arms for 16 weeks: 125 mg every four weeks, 250 mg every four weeks, or 250 mg every two weeks [4]. Evaluation of data analysis showed that lebrikizumab rapidly improved symptomatology and quality of life in a dose-dependent manner compared with placebo (e.g., EASI50, EASI75, pruritus scores). The side effect profile was favorable, and tolerability was generally good [1]. Phase III studies to further evaluate efficacy and safety are currently ongoing.
Other potential therapeutic options currently being tested in clinical trials are: Mepolizumab (anti-IL5), Nemolizumab (anti-IL31), TSLP-OX40/IL33 axis, anti-OSMR-beta, Fezakinumab (anti-IL22), Bermekimab (anti-IL1α), and the IgE inhibitors Omalizumab and Ligelizumab [1].
Summary
- The market approval of the IL4/IL13 inhibitor dupilumab (Dupixent®) is a milestone for the treatment of moderate to severe atopic dermatitis.
- Other biologics have shown promising results in clinical trials, including the IL13 inhibitors tralokinumab and lebrikizumab.
- In the “small molecule” setting, baricitinib has been shown to be effective and safe in phase III trials. JAK inhibitors are produced synthetically.
Literature:
- Maul JT: What’s new 2019/2020: inflammatory dermatoses. Julia-Tatjana Maul, MD, Zurich Dermatology Continuing Education Days (ZDFT), May 14-15, 2020.
- Guttman-Yassky E, et al: J Am Acad Dermatol. 2019; 80: 913-921.e9.
- Simpson EL, et al: Baricitinib in Patients With Moderate-To-Severe Atopic Dermatitis and Inadequate Response to Topical Corticosteroids: Results From Two Randomized Monotherapy Phase III Trials. Br J Dermatol 2020 Jan 5. doi: 10.1111/bjd.18898. online ahead of print.
- Loh TY, et al: Therapeutic Potential of Lebrikizumab in the Treatment of Atopic Dermatitis. J Asthma Allergy 2020; 13: 109-114.
- Brückmann H, Zhong S, Stark H: Pathogenesis and guideline-based therapy of atopic dermatitis.www.deutsche-apotheker-zeitung.de/daz-az/
- 2018/daz-31-2018/juckreiz-schuppen-roetung.
- Gross GE, et al: S2k guideline on the diagnosis and treatment of zoster and postzoster neuralgia. JDDG 2020; 18(1): 55-79.
- Petronelli M: Tralokinumab meets endpoints for all phase 3 studies, www.dermatologytimes.com, last accessed 07/01/2020.
DERMATOLOGY PRACTICE 2020; 30(4): 26-27