Sleep-disordered breathing is now a recognized cardiac risk factor. It can trigger not only circulatory diseases such as hypertension and heart failure, but also atrial fibrillation. As a result, it often goes undetected and insidiously jeopardizes patients’ prognosis.
In recent years, obstructive sleep apnea (OSA) has been shown to be a common disorder in adults. It is characterized by repeated apneas and hypoxia caused by collapse of the upper airway during sleep despite the respiratory effort of the diaphragm. Five or more apneas per hour of sleep are generally considered abnormal, severely affected patients have several hundred apneas per night. Most apneas and hypopneas are terminated by a transient awakening from sleep followed by hyperventilation.
Arterial hypertension and sleep apnea
Nocturnal arterial blood pressure is elevated in OSA patients, and there is growing evidence that OSA is also an independent risk factor for arterial hypertension during the day. Although the exact mechanisms are still unclear, persistent elevation of sympathetic tone caused by chronic repetitive hypoxia and arousal are thought to be the key mechanisms for the short- and long-term blood pressure elevations in OSA.
Nasal continuous positive airway pressure (nCPAP) has become the standard treatment for OSA and has been shown to reduce symptoms and improve quality of life in OSA patients. However, controlled studies showed either no effect or only a small reduction in arterial blood pressure of 1.4 or 2.5 mm Hg. The overall efficacy of this treatment on cardiovascular sequelae in OSA patients has been questioned, explains Priv.-Doz. Dr. Jan Börgel, St. Barbara Clinic Heessen Internal Medicine Hamm, Germany [1].
Antihypertensive effect of CPAP therapy
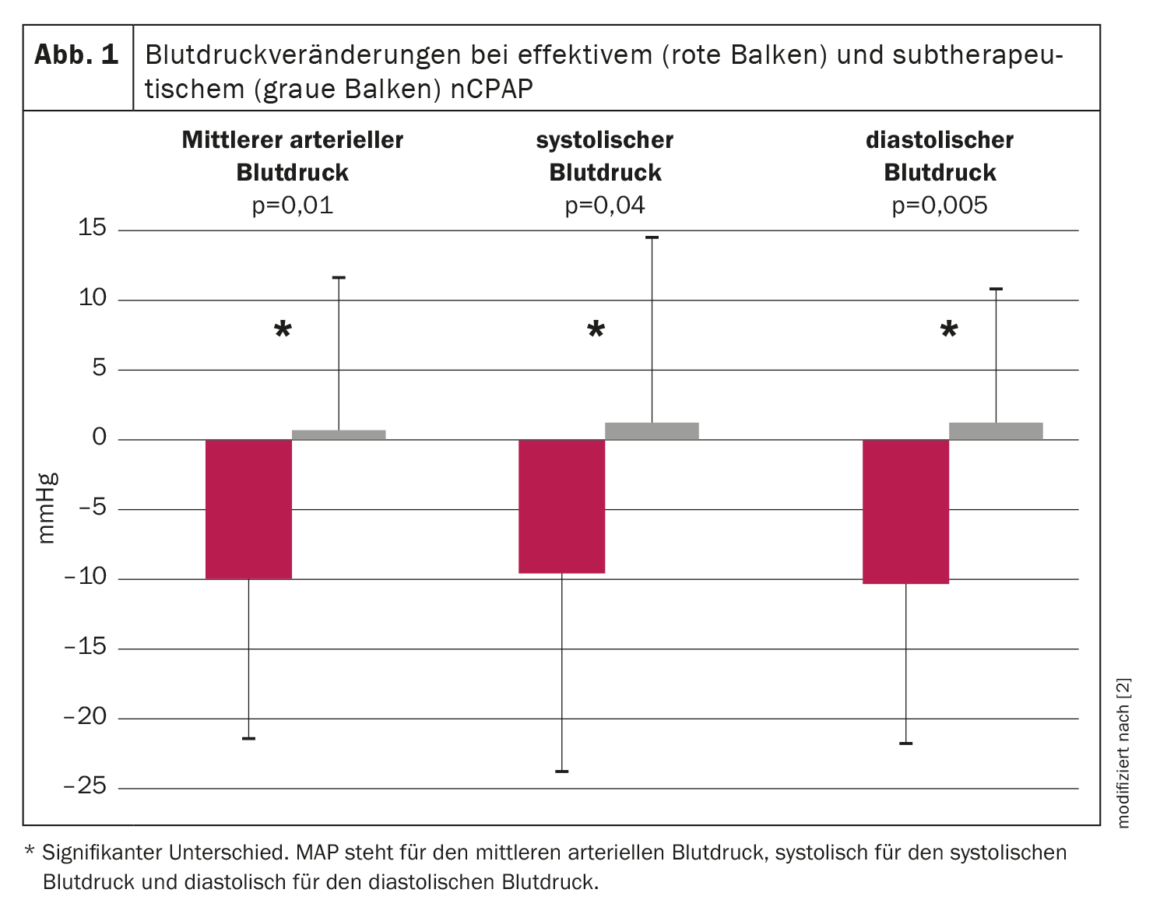
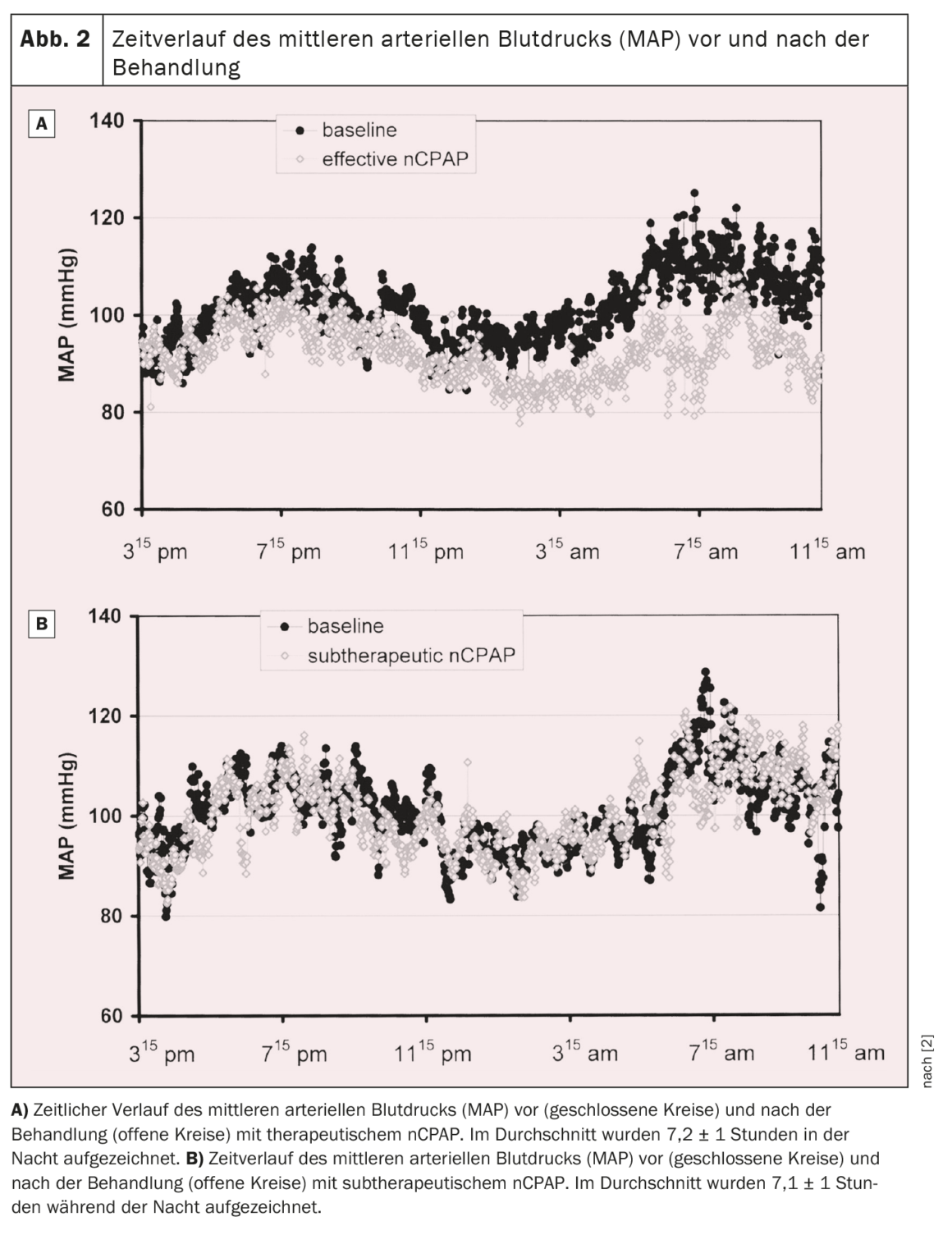
A prospective randomized trial was therefore conducted to investigate the effect of nCPAP on arterial blood pressure in OSA patients. A total of 60 consecutive patients with moderate to severe OSA were randomly assigned to either effective or subtherapeutic nCPAP treatment for an average of nine weeks. Nocturnal polysomnography and continuous noninvasive blood pressure recording for 19 hours were performed before and during treatment. Apneas and hypopneas were reduced by ≈95% and 50% in the therapeutic and subtherapeutic groups, respectively. Mean arterial blood pressure decreased during the 19.1 ± 1.3-hour recording period by 9.9 ± 11.4 mm Hg in the therapeutic nCPAP group, while it increased by 0.6 ± 10.8 mm Hg in the subtherapeutic nCPAP group (p=0.01, ANOVA interaction time by group). Both diastolic and systolic blood pressure also decreased significantly under therapeutic nCPAP by 10.3 ± 11.4 mm Hg and 9.5 ± 15.0 mmHg, respectively (p<0.005 and p=0.04, respectively, ANOVA interaction time by group) compared to subtherapeutic nCPAP (Fig. 1) . The decrease in mean blood pressure under effective nCPAP was observed both during the day (-10.0 ± 12.1 mmHg) and at night (-10.3 ± 15.3 mmHg). The time course of mean arterial blood pressure before and during treatment for both groups is shown in Figure 2 [2]. In the group with effective treatment, mean arterial blood pressure decreased throughout the recording period, with the greatest decrease occurring at night and in the morning until about noon. In the afternoon and evening, the drop in blood pressure was still present, but less pronounced than at night and in the first half of the day [2].
A meta-analysis of randomized controlled trials (RCTs) continued to quantify the effect size of blood pressure reduction with CPAP therapy compared with other passive (sham CPAP, placebo tablets, conservative measures) or active treatments (swallow, antihypertensive medications). Of 1,599 articles, 31 RCTs were included that compared CPAP with either passive or active treatment. In random effects analysis compared to passive treatment (29 RCTs, 1820 subjects), a mean ± SEM net difference in systolic blood pressure of 2.6 ± 0.6 mmHg and diastolic blood pressure of 2.0 ± 0.4 mmHg was found, favoring CPAP treatment (p<0.001). In the studies with 24-hour ambulatory blood pressure monitoring that presented data over the daytime and nighttime periods, the mean difference in systolic and diastolic blood pressure was 2.2 ± 0.7 and 1.9 ± 0.6 mmHg, respectively, during the day and 3.8 ± 0.8 and 1.8 ± 0.6 mmHg, respectively, during the night. In regression analysis, a higher apnea/hypopnea index at baseline was associated with a greater mean net decrease in systolic blood pressure (β ± SE, 0.08 ± 0.04). The results showed that CPAP therapy significantly reduced blood pressure in patients with OSA, but with a small effect size. Patients with frequent apnea episodes may benefit most from therapy [3].
Another study analyzed the effect of CPAP on blood pressure (BP) in patients with OSA and resistant hypertension. RCTs that examined the effect of CPAP on blood pressure in patients with OSA and resistant hypertension and were indexed in MEDLINE, Embase, and the Cochrane Library from inception to March 20, 2015, were included. A total of five RCTs were identified that met the inclusion criteria. Pooled changes after CPAP treatment for 24-hour ambulatory systolic and diastolic blood pressure (DBP) were -4.78 mm Hg (95% confidence interval [CI], -7.95 to -1.61) and -2.95 mmHg (95% CI, -5.37 to -0.53) in favor of the CPAP group. CPAP was also associated with a reduction in nocturnal DBP (mean difference, -1.53 mmHg, 95% CI, -3.07-0). These results suggest a favorable reduction in blood pressure with CPAP treatment in patients with OSA and resistant hypertension [4].
Hypoxic stress predicts CVD-associated mortality.
Still controversial to date has been the impact of OSA on fatal CVD events, as associations between exposure and outcome have been inconsistent. Recent evidence suggested that there is no association between positive airway pressure therapy and secondary CVD prevention.
To date, sleep medicine has relied on quantifying the frequency of apneas and hypopneas observed during sleep (apnea-hypopnea index, AHI) to diagnose OSA and determine its severity. Based on these metrics, OSA was found to modestly predict mortality, but the results were largely limited to younger to middle-aged men. It has been questioned whether the AHI captures the most important aspects of OSA that adversely affect the cardiovascular system. Obstructive sleep apnea is a condition in which repeated airway obstruction impairs ventilation and causes blood gas levels to be disturbed. Therefore, the AHI, which is a simple count of obstructive episodes per hour of sleep without consideration of the duration and depth of ventilatory disturbance or blood gas changes, is not a complete description of physiologic disturbances. Several observational studies have shown that measures of nocturnal hypoxemia, such as the percentage of time during sleep with oxygen saturation below 90% (TST90), are more predictive of CVD and all-cause mortality than AHI. However, the TST90 and similar measures characterize not only intermittent hypoxemia secondary to obstructive events, but also persistent hypoxemia, e.g., due to chronic obstructive pulmonary disease (COPD) or hypoventilation in obesity unrelated to upper airway obstruction and OSA.
One study therefore sought to develop a measure of OSA severity that quantified OSA-related hypoxemia, which was hypothesized to significantly predict CVD-related mortality after adjustment for routinely measured polysomnography (PSG) indices. Accordingly, a measure was developed to capture the frequency, duration, and depth of the contribution of respiratory events to arterial hypoxemia, specifically the “area under the curve” of oxygen desaturation associated with individual apneas and hypopneas-the OSA-specific “hypoxic burden.”
The samples were from two cohort studies: the Outcomes of Sleep Disorders in Older Men. (MrOS), which included 2743 men aged 76.3 ± 5.5 years, and the Sleep Heart Health Study (SHHS), which included 5111 middle-aged and older adults (52.8% women) aged 63.7 ± 10.9 years. Outcomes included all-cause mortality and cardiovascular disease (CVD)-related mortality. Hypoxia stress was determined by measuring the area under the final saturation curve compared with the baseline situation before the event. Cox models were used to calculate the adjusted risk ratios for hypoxic stress. Unlike AHI, “hypoxic stress” strongly predicted CVD mortality and all-cause mortality (MrOS only). Subjects in the MrOS trial with hypoxia exposure in the two highest quintiles had hazard ratios of 1.81 [95% confidence interval (CI) 1.25-2.62] and 2.73 (95% CI 1.71-4.36), respectively. Similarly, the group in the SHHS with “hypoxic stress” in the highest quintile had a hazard ratio of 1.96 (95% CI 1.11-3.43). Accordingly, “hypoxic load,” an easily derived signal from the overnight sleep study, predicts CVD mortality in different populations. The results suggest that not only the frequency but also the depth and duration of sleep-related upper airway obstruction are important disease characterizing features [5].
The excessively sleepy phenotype was identified as a predictor of new-onset and recurrent cardiovascular events, although not of cardiovascular mortality. Nevertheless, even in clinical trials that focused on participants with minimal symptoms or no drowsiness, limited benefits of OSA treatment on blood pressure and cardiovascular outcomes were found [6].
Blood pressure lowering – Dipper status in untreated OSA/hypertension.
A post-hoc analysis examined the effect of CPAP on blood pressure considering the circadian blood pressure pattern in untreated hypertensive patients. Subjects were classified based on the dipping ratio (dipper/non-dipper). A total of 272 hypertensive patients were included in the analysis (113 dippers and 159 nondippers). Baseline clinical and polysomnographic variables were similar in both groups. CPAP treatment of nondipper patients was associated with reductions in 24-hour ambulatory blood pressure variables and nocturnal ambulatory blood pressure measurements. However, a non-significant effect was found in the dipper group. The differential effects of CPAP between groups were -2.99 mmHg (95% CI -5.92 to -0.06 mmHg) for mean 24-hour ambulatory blood pressure and -5.35 mmHg (95% CI -9.01 to -1.69 mmHg) for mean nocturnal ambulatory blood pressure. The results show a differential effect of CPAP treatment on blood pressure in hypertensive patients depending on the circadian pattern. According to this study, only non-dipper patients benefited from CPAP treatment in terms of blood pressure reduction [7].
Atrial fibrillation and sleep apnea
OSA not only causes hypoxemia, hypercapnia, autonomic dysfunction, agitation, and significant negative intrathoracic pressure changes, explains Prof. Dr. Dr. Anil-Martin Sinha, Sana Klinikum Hof GmbH Clinic for Cardiology, Nephrology, Pneumology and Internal Intensive Care Medicine Hof, Germany [8], the pathophysiology of OSA also leads to inflammation, endothelial dysfunction, imbalanced coagulation, hemodynamic changes, electrical/structural remodeling of the atria/ventricles, and autonomic dysregulation. These factors are associated with the development and maintenance of atrial fibrillation. Thus, the pathophysiology of OSA is multifactorial, and many unresolved complex mechanisms are involved in the development of AF, with both acute and long-term implications for arrhythmogenic substrates [9].
GJA1 expression and left atrial remodeling.
Studies have shown that genes controlling inflammation, gap junctions, and atrial fibrosis are associated with the pathophysiological mechanism of AF. In humans, the connexin-43 protein is encoded by the GJA1 gene on chromosome 6 and is expressed by atrial and ventricular cardiomyocytes, vascular smooth muscle cells, endothelial cells, monocytes, and macrophages. Myocardial electrical continuity is maintained by connexins located in gap junctions that maintain low resistance intercellular coupling. Differences in connexin-43 expression result in inconsistent discontinuous conduction and cardiac arrhythmias. Exosomes, membrane-bound vesicles 40-100 nm in diameter, are released by many cell types including blood cells, endothelial cells, immune cells, platelets, and smooth muscle cells and are present in almost all biological fluids. RNAs in exosomes can be taken up by neighboring or distant cells when exosomes circulate and subsequently modulate recipient cells. The discovery of their function in genetic exchange between cells has brought exosomes increasing attention.
One study investigated the predictors of AF occurrence in patients with OSAS and the effects of exosomes from OSAS patients with and without AF on the expression of GJA1 and other inflammatory and fibrosis genes involved in the pathophysiology of AF in HL-1 cells to elucidate their association with AF occurrence. The study provided several important findings. First, OSAS patients with AF had more diabetes mellitus, lower sleep efficiency, lower LVEF, and larger left atrium (LA) than OSAS patients without AF. Second, left atrial size was the most significant predictor of the occurrence of AF in OSAS patients, with a cutoff value of 38.5 mm. Third, mRNA gene expression of GJA1 was lower and TNF-α was higher in HL-1 cells incubated with exosomes from OSAS patients with AF than in those incubated with exosomes from OSAS patients without AF. After controlling for age and sex, gene expression of GJA1 was still lower in HL-1 cells incubated with exosomes from OSAS patients with AF. Finally, GJA1 gene expression was negatively correlated with AHI and oxygen desaturation index in OSAS patients with AF, especially during the non-REM phase [10].
Prevalence of undiagnosed sleep apnea in atrial fibrillation.
The aim of another study was to determine the proportion of patients with atrial fibrillation (AF) who also had undiagnosed sleep apnea and to examine the impact of this diagnosis on adherence to sleep apnea therapy. The prospective study included 188 consecutive patients with atrial fibrillation without a prior diagnosis of sleep apnea who were scheduled for atrial fibrillation ablation. Home sleep apnea testing was positive in 155 of 188 patients (82.4%); of these 155, 127 (82%) had a predominantly obstructive component and 28 (18%) had mixed sleep apnea with a central component of 15.2 ± 7.4%. The severity of sleep apnea was mild in 43.8%, moderate in 32.9%, and severe in 23.2%. The sensitivity and specificity of the STOP-BANG questionnaire were 81.2% and 42.4%, respectively. In multivariate analysis, STOP-BANG was not predictive of sleep apnea (odds ratio: 0.54; 95% confidence interval: 0.17-1.76; p=0.31). Therapy with continuous positive airway pressure ventilator was initiated in 73 of 85 patients (85.9%) with moderate or severe sleep apnea, and 68 of the 73 patients (93.1%) remained symptom-free after a mean follow-up of 21 ± 6.2 months. Accordingly, sleep apnea is extremely common in patients with AF referred for ablation, a large proportion of whom are undiagnosed because the predictive power of sleep apnea symptoms in this group of AF patients is limited. Screening for sleep apnea resulted in a high rate of patients adhering to continuous positive airway pressure in the long term [11].
Effects of CPAP on the atrial fibrillation substrate.
The SLEEP-AF trial investigated how treatment of OSA affects the atrial substrate in AF. For this purpose, 24 consecutive patients with at least moderate OSA (AHI ≥15) referred for treatment of AF were recruited. Participants were randomized 1:1 to receive either continuous positive airway pressure (CPAP) or no therapy (n=12 with CPAP; n=12 without CPAP). All participants underwent invasive electrophysiological testing (high-density mapping of the right atrium) at baseline and after at least six months. Outcome variables were atrial voltage (mV), conduction velocity (m/s), atrial area <0.5 mV (%), percentage of complex points (%), and effective atrial refractory period (ms). Clinical features and electrophysiological parameters were similar in the two groups at baseline. Adherence to CPAP therapy was high (device use: 79% ± 19%; mean use/day: 268 ± 91 min) and resulted in a significant reduction in AHI (mean reduction: 31 ± 23 events/h). There were no differences between the groups in blood pressure and body mass index over time. At follow-up, the CPAP group had higher conduction velocity (0.86 ± 0.16 m/s vs. 0.69 ± 0.12 m/s; p (time × group) = 0.034), significantly higher voltages (2.30 ± 0.57 mV vs. 1.94 ± 0.72 mV; p<0.05), and a lower percentage of complex points (8.8% ± 3.61% vs. 11.93% ± 4.94%; p=0.011) compared to the control group. CPAP therapy also tended to result in a lower percentage of atrial surface <0.5 mV (1.04% ± 1.41% vs. 4.80% ± 5.12%; p=0.065). The results show that CPAP therapy leads to reversal of atrial remodeling in AF and provides mechanistic evidence supporting the treatment of OSA in AF [12].
Relationship between ICD detected sleep apnea and atrial fibrillation.
The Respiratory Disturbance Index (RDI) calculated by an implantable cardioverter defibrillator (ICD) algorithm enables accurate detection of severe sleep apnea (SA). A recent study has now tested whether the RDI can also predict atrial fibrillation burden. The weekly mean RDI calculated throughout the follow-up period and over a period of one week before the sleep study was considered. Severe AF (RDI ≥30 episodes/h) was diagnosed in 92 (56%) patients at the time of the sleep study. During follow-up, AF burden ≥5 minutes/day was documented in 70 (43%), ≥6 hours/day in 48 (29%), and ≥23 hours/day in 33 (20%) patients. Device-recorded RDI ≥30 episodes/h at the time of polygraphy and apnea-hypopnea index ≥30 episodes/h measured with polygraphy were not associated with the occurrence of the end points using a Cox regression model. However, using a time-dependent model, a continuously measured weekly mean RDI ≥30 episodes/h was independently associated with AF exposure ≥5 min/day (hazard ratio [HR]: 2.13, 95% confidence interval [CI]: 1.24-3.65, p=0.006), ≥6 h/day (HR: 2.75, 95% CI: 1.37-5.49, p=0.004) and ≥23 h/day (HR: 2.26, 95% CI: 1.05-4.86, p=0.037) were associated. The results show that heart failure patients with ICD-diagnosed severe AF are two- to threefold more likely to experience an AF episode, depending on the threshold of daily AF burden [13].
Heart failure and sleep apnea
Sleep disturbances are common in patients with heart failure with reduced ejection fraction (HFrEF), with prevalence rates reported to be 50% to 75%. In particular, OSA occurs more frequently in patients with heart failure than in the general population. Central sleep apnea, which may manifest as Cheyne-Stokes breathing, is seen in 25% to 40% of patients with heart failure with decreased ejection fraction. Thereby, the prevalence of central sleep apnea increases in parallel with the increasing severity of heart failure and the worsening of cardiac dysfunction, explains Prof. Dr. Michael Arzt, University Hospital Regensburg Clinic and Polyclinic for Internal Med. II, Cardiology Regensburg, Germany [14]. There are a number of mechanisms by which central sleep apnea can adversely affect cardiac function, including increased sympathetic nervous system activity and intermittent hypoxemia. In addition, central sleep apnea is an independent risk marker for poor prognosis and death in patients with heart failure.
In the CanadianContinuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure (CANPAP) study, patients with heart failure and central sleep apnea were randomized to receive either CPAP or no CPAP. The study was stopped early and showed no beneficial effect of CPAP on morbidity or mortality. A post-hoc analysis suggested that mortality might be lower if the apnea-hypopnea index (AHI; the number of apnea or hypopnea events per hour of sleep) is reduced to less than 15 events per hour [15].
Effects of adaptive servo ventilation
Both types of sleep-disordered breathing, obstructive and central sleep apnea (OSA and CSA, respectively), are common in patients with heart failure and reduced ejection fraction (HFrEF). To date, it has been unclear whether treatment of sleep-disordered breathing with adaptive servoventilation (ASV) reduces morbidity and mortality in these patients. Adaptive servoventilation is a noninvasive ventilation therapy that effectively relieves central sleep apnea by providing servo-controlled inspiratory pressure support in addition to expiratory positive airway pressure.
The SERVE-HF study (Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure). investigated the effects of ASV (AutoSet CS, ResMed) on survival and cardiovascular outcomes in patients with heart failure with reduced ejection fraction and predominantly central sleep apnea who were treated in addition to guideline-required medical treatment. A total of 1325 patients with a left ventricular ejection fraction of 45% or less, an apnea-hypopnea index (AHI) of 15 or more events (apnea or hypopnea events) per hour, and a predominance of central events were randomly assigned to guideline-based medical treatment with ASV or guideline-based medical treatment alone (control). The primary end point in the time-to-event analysis was the first death event from any cause, a life-saving cardiovascular intervention (heart transplantation, implantation of a cardiac assist device, resuscitation after sudden cardiac arrest, or an appropriate life-saving shock), or unplanned hospitalization for worsening heart failure.
In the group with ASV, the mean AHI at 12 months was 6.6 events per hour. The incidence of the primary endpoint was not significantly different between the ASV-ventilated group and the control group (54.1% and 50.8%, respectively; hazard ratio, 1.13; 95% confidence interval [CI], 0.97-1.31; p=0.10). All-cause mortality and cardiovascular mortality were significantly higher in the group with ASV than in the control group (hazard ratio for death from any cause, 1.28; 95% CI, 1.06-.55; p=0.01; and hazard ratio for cardiovascular death, 1.34; 95% CI, 1.09-1.65; p=0.006). ASV thus had no significant effect on the primary end point in patients with heart failure with reduced ejection fraction and predominantly central sleep apnea, but both all-cause and cardiovascular mortality were increased with this therapy [16]. Several explanations have been proposed for how ASV may trigger ventricular arrhythmias, such as rapid changes in blood gases, pH, and potassium levels, and effects on venous return and transmural wall tension from applied positive airway pressure.
Therefore, an ancillary analysis of the main SERVE-HF substudy evaluated the impact of ASV on the burden of nocturnal ventricular arrhythmias in ASV-treated patients with HFrEF and central sleep apnea. From study entry to 3- and 12-month follow-up, the number of premature ventricular complexes (control: median 19.7, 19.0, and 19.0; ASV: 29.1, 29.0, and 26.0 events/h; p=0.800) and the occurrence of ≥1 noncontinuous ventricular tachycardia/night (control: 18, 25, and 18% of patients; ASV: 24, 16, and 24% of patients; p=0.095) were similar in the control and ASV groups. The addition of ASV to guideline-required medical treatment had no significant effect on nocturnal ventricular ectopy or tachyarrhythmias over a 12-month period in living patients with HFrEF and central sleep apnea. The results do not support the hypothesis that ASV can lead to sudden cardiac death by triggering ventricular tachyarrhythmias [17].
In contrast, the ADVENT-HF trial, a multicenter, multinational, randomized, open-label, parallel-group study with blinded assessment of the end points of standard medical therapy for HFrEF alone versus the additional administration of ASV in patients with HFrEF and sleep-disordered breathing, failed to demonstrate either a positive or a negative effect of ASV therapy on prognosis in patients with HFrEF (LVEF <45%) and obstructive or central sleep apnea [18].
Sleep-related respiratory disorders not limited to HFrEF patients only
Currently available data on sleep-disordered breathing in heart failure focus primarily on HFrEF, whereas data on sleep-disordered breathing and heart failure with preserved ejection fraction (HFpEF) are limited. However, moderate to severe sleep-disordered breathing also appears to be a common comorbidity in HFpEF, affecting 37-58% of patients. For example, treatment of OSA in patients with HFpEF offers the opportunity to improve quality of life and physical performance and has the potential to prevent progression of HFpEF by lowering arterial blood pressure and cardiac workload and preventing cardiac remodeling. Therefore, due to the different pathophysiology, prognostic impact and implications, and treatment modalities, it is crucial to distinguish between HF patients with predominant OSA or CSA.
Therefore, the aim of the analysis of the SleepHF-XT registry was to investigate the sex-specific prevalence and predictors of sleep-disordered breathing (both OSA and CSA) in patients with HFpEF compared with those with mildly reduced ejection fraction (HFmrEF) or HFrEF. Of the 3289 patients included, 2032 had HFpEF, 559 had HFmrEF, and 698 had HFrEF. The prevalence of sleep-related breathing disorders was high in HFpEF but significantly lower than in HFmrEF or HFrEF (36% versus 41 and 48%, respectively). The rates of sleep-disordered breathing in men and women were 41 and 28% for HFpEF, 44 and 30% for HFmrEF, and 50 and 40% for HFrEF. The proportion of men and women with sleep-disordered breathing who had OSA was significantly higher in HFpEF than in HFrEF. Male sex, older age, higher body mass index, and New York Heart Association functional class III/IV were significant predictors of moderate to severe sleep-disordered breathing in HFpEF patients. Thus, the prevalence of sleep-disordered breathing in HFpEF was high but lower than in patients with HFmrEF or HFrEF. Moderate to severe sleep-disordered breathing occurred more frequently in men than in women across the spectrum of heart failure. In both sexes, the proportion of OSA with HFpEF was higher than those with HFrEF [19].
Patient clusters can support clinical decision making
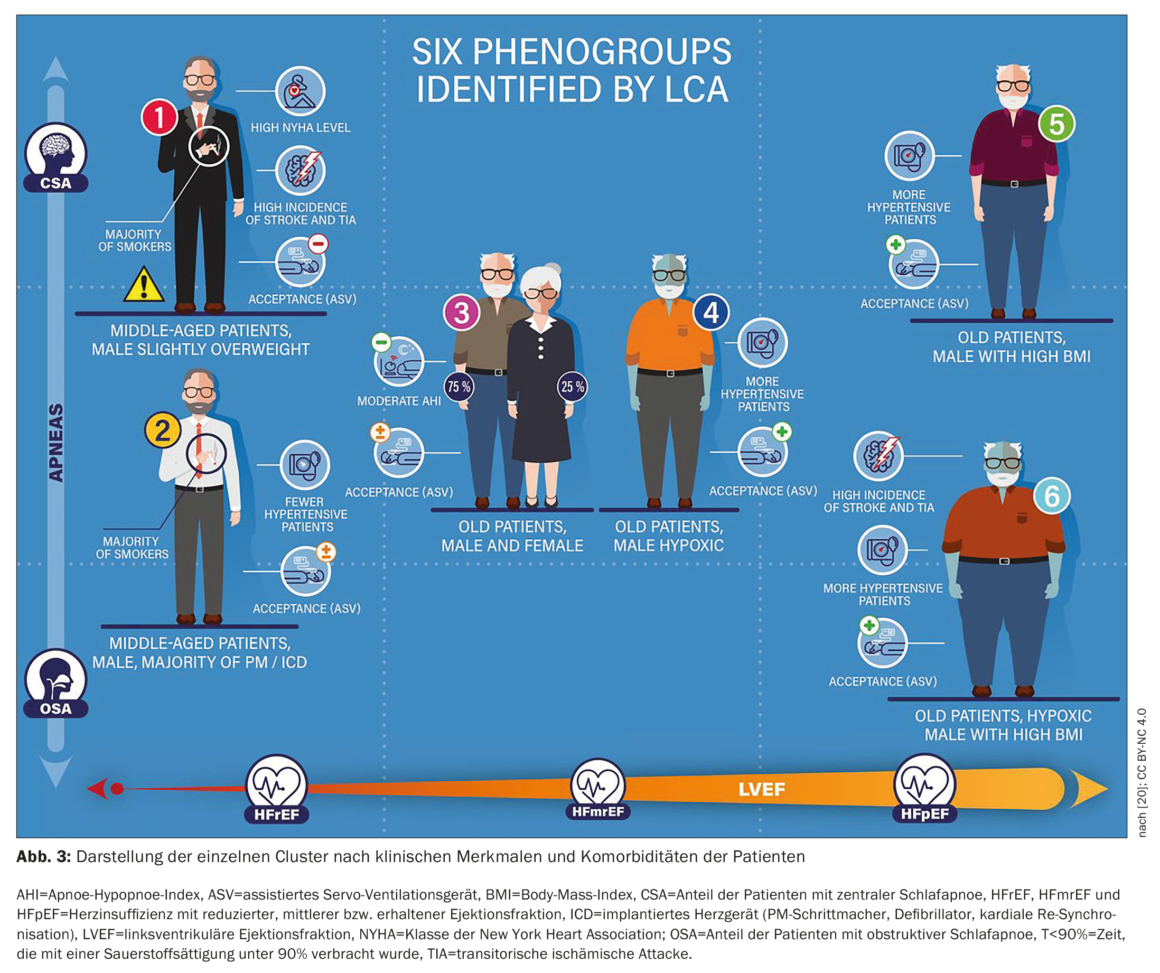
The FACE study, a European, multicenter, prospective observational cohort study, used real-world data to evaluate the effect of PAP therapy with ASV on morbidity and mortality in patients with HFrEF, HFmrEF, or HFpEF and central sleep-disordered breathing or coexisting CSA/OSA. Three-month data in patient subgroups defined using latent class analysis (LCA) were presented. The LCA identified, for the first time, six discrete patient groups that represent clinically relevant subgroups related to SDB management in heart failure patients with varying ASV utilization and prognosis. The 3-month rate of primary endpoints was significantly higher in patients from cluster 1 (predominantly men, low LVEF, severe HF, CSA; 13.9% vs. 1.5-5% in the other clusters, p<0.01). This may improve patient phenotyping in clinical practice and allow individualization of therapy.
Figure 3 [20] shows the clinical representation of each cluster. The main parameters that distinguished between clusters were obstructive AHI, central AHI, LVEF (%), NYHA class I/II, T90, and the presence of HFrEF. As expected, there were significant differences in patient demographics, HF disease characteristics, and SDB characteristics between clusters resulting directly from the LCA methodology. Interestingly, ASV adherence and ASV refusal differed significantly between clusters, although these variables were not included in the LCA model. The 3-month rate of primary endpoints was significantly higher in patients from cluster 1 (predominantly men, low LVEF, severe HF, CSA; 13.9% vs. 1.5-5% in the other clusters, p<0.01). This cluster corresponded to those with a poor prognosis under ASV therapy in the SERVE-HF enrolled population [20].
Congress: 89th Annual Meeting of the DGK
Literature:
- Börgel J: Neues vom unheilvollen Duo Nr. 1: Arterielle Hypertonie und Schlafapnoe. 89. Jahrestagung der DGK, 12.04.2023, Sitzung: Kardiovaskuläre Erkrankungen und schlafbezogene Atmungsstörungen im digitalen Zeitalter.
- Becker HF, et al.: Effect of Nasal Continuous Positive Airway Pressure Treatment on Blood Pressure in Patients With Obstructive Sleep Apnea. Circulation 2002; https://doi.org/10.1161/01.CIR.0000042706.47107.7A.
- Fava C, et al.: Effect of CPAP on Blood Pressure in Patients With OSA/Hypopnea: A Systematic Review and Meta-analysis. ScienceDirect 2014; https://doi.org/10.1378/chest.13-1115.
- Liping L, et al.: Continuous Positive Airway Pressure in Patients With Obstructive Sleep Apnea and Resistant Hypertension: A Meta-Analysis of Randomized Controlled Trials. J Clin Hypertens (Greenwich) 2016 Feb; doi: 10.1111/jch.12639.
- Azarbarzin A, et al.: The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019; doi: 10.1093/eurheartj/ehy624.
- Mehra R, Azarbarzin A: Sleep Apnea–Specific Hypoxic Burden and Not the Sleepy Phenotype as a Novel Measure of Cardiovascular and Mortality Risk in a Clinical Cohort. Am J Respir Crit Care Med 2022; doi: 10.1164/rccm.202110-2371ED.
- Sapiña-Beltrán E, et al.: Differential blood pressure response to continuous positive airway pressure treatment according to the circadian pattern in hypertensive patients with obstructive sleep apnoea. European Respiratory Journal 2019;
doi: 10.1183/13993003.00098-2019. - Sinha AM: Neues vom unheilvollen Duo Nr. 2: Vorhofflimmern und Schlafapnoe. 89. Jahrestagung der DGK, 12.04.2023, Sitzung: Kardiovaskuläre Erkrankungen und schlafbezogene Atmungsstörungen im digitalen Zeitalter.
- Iwasaki Y: Mechanism and management of atrial fibrillation in the patients with obstructive sleep apnea. Journal of Arrhythmia 2022;
https://doi.org/10.1002/joa3.12784. - Chen YL, et al.: GJA1 Expression and Left Atrial Remodeling in the Incidence of Atrial Fibrillation in Patients with Obstructive Sleep Apnea Syndrome. Biomedicines 2021; doi: 10.3390/biomedicines9101463.
- Shapira-Daniels A, et al.: Prevalence of Undiagnosed Sleep Apnea in Patients With Atrial Fibrillation and its Impact on Therapy. JACC Clin Electrophysiol 2020; doi: 10.1016/j.jacep.2020.05.030
- Nelliah CJ, et al.: Impact of CPAP on the Atrial Fibrillation Substrate in Obstructive Sleep Apnea: The SLEEP-AF Study. JACC Clin Electrophysiol 2022; doi: 10.1016/j.jacep.2022.04.015.
- Boriani G, et al.: Association between implantable defibrillator-detected sleep apnea and atrial fibrillation: The DASAP-HF study. J Cardiovasc Electrophysiol 2022; doi: 10.1111/jce.15506.
- Arzt M: Neues vom unheilvollen Duo Nr. 3: Herzinsuffizienz und Schlafapnoe. 89. Jahrestagung der DGK, 12.04.2023, Sitzung: Kardiovaskuläre Erkrankungen und schlafbezogene Atmungsstörungen im digitalen Zeitalter.
- Bradley TD, et al.: Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; doi: 10.1056/NEJMoa051001.
- Cowie MR, et al.: Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med 2015; doi: 10.1056/NEJMoa1506459.
- Fisser C, et al.: Effects of Adaptive Servo-Ventilation on Nocturnal Ventricular Arrhythmia in Heart Failure Patients With Reduced Ejection Fraction and Central Sleep Apnea-An Analysis From the SERVE-HF Major Substudy. Front Cardiovasc Med 2022; doi: 10.3389/fcvm.2022.896917.
- Lyons OD, et al.: Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail 2017; doi: 10.1002/ejhf.790; Bradley D, et al.: ESC Congress 2022.
- Arzt M, et al.: Prevalence and predictors of sleep-disordered breathing in chronic heart failure: the SchlaHF-XT registry. ESC Heart Fail 2022;
doi: 10.1002/ehf2.14027. - Tamisier R, et al.: Adaptive servo ventilation for sleep apnoea in heart failure: the FACE study 3-month data. Thorax 2022; doi: 10.1136/thoraxjnl-2021-217205.
CARDIOVASC 2023; 22(2): 42–47