The results of the international multicenter study APPRECIATE show a significant symptom reduction including skin involvement of specific sites. In addition, a significant improvement in quality of life was measurable.
The companies participating in the 101. The data for the Swiss subsample (n=83) presented at the 12th Annual Meeting of the SGDV demonstrate the efficacy of OTEZLA® (apremilast) in this heterogeneous patient subpopulation. The characteristic specific and highly distressing disease manifestations, such as itching or involvement of the scalp, nails, and palms and soles, can severely impact quality of life. Comorbidities as possible sequelae lead to an increase in the “Burden of Disease” and the complexity of the disease. Subjective assessment of severity is often not congruent with objective clinical assessment. “Certain manifestations of psoriasis can have a major impact on the quality of life of those affected. In patients with limited skin involvement, but who have itching or scalp, nail, palm and plantar involvement, topical treatments, phototherapy or conventional systemic treatments sometimes fail to adequately alleviate the burden of the disease,” said Professor Curdin Conrad, University Hospital Lausanne (CHUV).
Benefit assessment in everyday clinical practice
The APPRECIATE study is a multinational, retrospective, cross-sectional observational study of adults with psoriasis treated with the oral phosphodiesterase-4 inhibitor OTEZLA® under everyday conditions. A total of 480 patients from 87 study centers in six European countries (Germany, Ireland, Austria, Sweden, Switzerland, United Kingdom) participated. The aim was to investigate patient characteristics and treatment outcomes, and to assess the benefits and limitations from the perspective of patients and physicians under everyday conditions [1]. The mean age of the study participants was 51.3 years, and the mean duration of illness was 18.6 years. Follow-up data collection took place 6(±1) months after treatment initiation. Therapy with OTEZLA® was performed according to standard clinical practice. Questionnaire data were collected from patients and physicians during routine clinical appointments. Descriptive statistics were used to analyze the pooled data of all patients and physicians at each study site [3]. Real-world data are becoming increasingly important in the comprehensive assessment of medicines, especially in a complex and multifaceted disease such as psoriasis.
Positive effects on skin lesions and quality of life
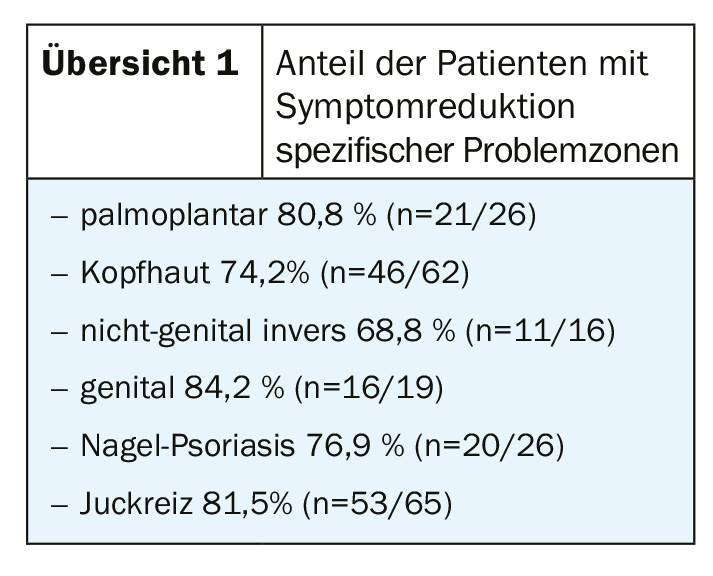
In Switzerland, a total of 83 patients at 16 study centers from all parts of the country were included in the study [1,2]. Analysis of the follow-up data set of the Swiss subsample (n=83) showed a significant symptom reduction (reduction of the PASI score to <3) in more than half of the study participants (52.2%) 6±1 months after the start of treatment [1]. Improvements in symptom expression were also observed when specific localizations were affected [1] (overview 1). Quality of life, measured by DLQI (Dermatology Life Quality Index), was also shown to improve [1]. While the mean DLQI at the start of OTEZLA® treatment was 14.4 (n=18) – corresponding to a very strong impact on disease-related quality of life – this value was reduced to a DLQI score of 5 or less in every second study participant after six months of treatment. The adverse events described were consistent with the known safety profile of OTEZLA® and specifically related to diarrhea (13.3%), nausea (12.1%), and headache (7.2%) [1]. Treatment was characterized by high persistence: 80% of patients maintained therapy after 6(±1) months of treatment. The data from the Swiss subsample of the APPRECIATE study are coherent with the results of the datasets from other European countries participating in the study [3].
Source: Celgene
Literature:
- Yawalkar N, et al.: Real-world experience with apremilast in Swiss patients with psoriasis: Analysis of physician-assessed outcomes from the APPRECIATE study. Presented at SGDV. 19-20 September 2019.
- Heidemeyer K, et al.: Real-world experience with apremilast in Swiss patients with psoriasis: Patient-reported insights from the APPRECIATE study. Presented at SGDV. 19-20 September 2019.
- Augustin M, et al: Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. Presented at the World Congress of Dermatology. 10-15 June 2019. Milan, Italy.
DERMATOLOGIE PRAXIS 2019; 29(6): 40 (published 8/12/19, ahead of print).