CGRP* antibodies for the prevention of migraine attacks, which have been approved since 2018, offer affected patients the possibility of a reduction in their headache days. Erenumab was the first of the monoclonal antibodies to receive approval. In the meantime, we can look back on experience gained over a period of more than one and a half years for practical use in everyday clinical practice.
* Calcitonin Gene-Related Peptide
Dr. Carl Göbel from the Department of Neurology, University Hospital Schleswig-Holstein, Lübeck Campus (D) and his colleagues from the Neurological-Behavioral Pain Clinic Kiel (D) have continuously evaluated the efficacy and tolerability of erenumab since November 2018 by analyzing data from the practice information system [1]. Substantial additional benefit was thus derived for adult patients who did not respond to, were not suitable for, or could not tolerate any of the approved drug therapies/classes of drugs.
A total of 193 patients aged 18-75 years were evaluated. The mean age was 47 years. 87.6% were women (n=169), 12.4% were men (n=24). On average, patients suffered from migraine on 16.9 days per month (minimum 7, maximum 30) and took acute medications on 12.4 days per month. The median duration of use of erenumab was 4.4 months. The 50% responder rate in terms of decrease in migraine days per month was 37.8% on average for all patients (women 40.2%, men 20.8%). There was an average decrease of 31.8% in migraine days per month. The decrease in triptan use days for the month was 34.5%. A reduction of migraine days per month by 30% was achieved by a mean of 55.4% of patients (Fig. 1).
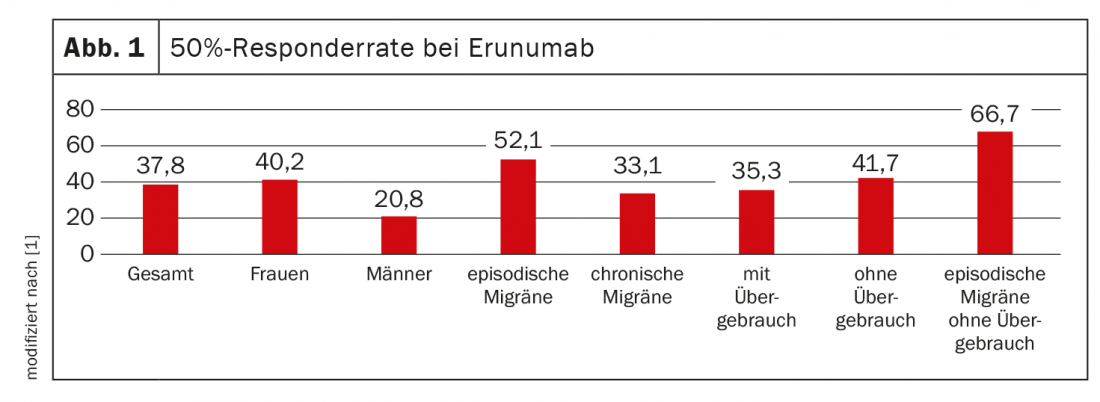
For episodic migraine, the 50% responder rate was 52.1% (n=48), and for chronic migraine, 33.1% (n=145). When acute medications were taken more than 10 days per month, the responder rate was 35.3% (n=113). These patients had undergone prior medication interruption with no significant reduction in headache days per month. If patients did not meet criteria for medication overuse, the responder rate was 41.7% (n=79). The highest 50% responder rate of 66.7% was achieved by patients with episodic migraine without medication overuse (n=24). The most common side effect was constipation (16.2%). 37.3% of patients discontinued therapy because of insufficient efficacy or tolerability.
Erenumab can bring about significant improvement in previously therapy-refractory patients, is Dr. Göbel’s conclusion. The effect was most pronounced in patients with episodic migraine and in patients without medication overuse, he said. Clinical experience confirmed the additional benefit, particularly in patients who had previously been refractory to therapy and who were largely excluded from the pivotal studies.
62.2% Erenumab non-responders
CGRP ligand antibodies represent an alternative for those patients who do not respond to erenumab. Again, researchers analyzed practice data in a specialized migraine and headache center to determine whether and to what extent erenumab non-responders may benefit from switching [2].
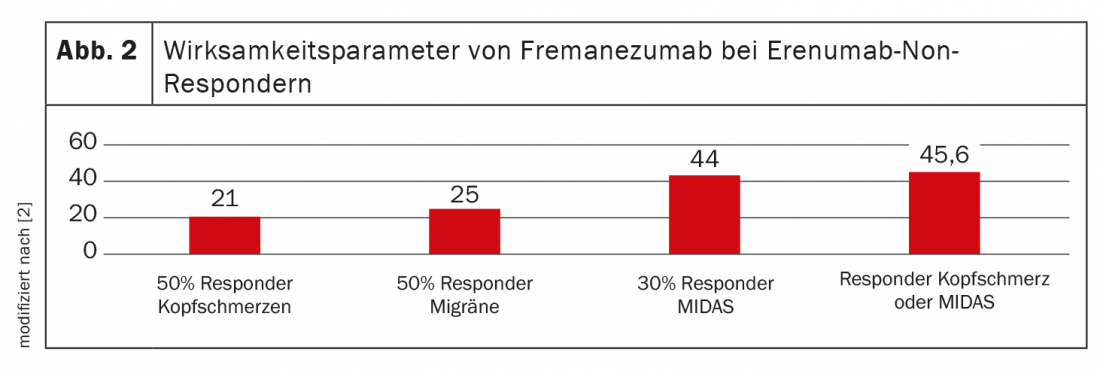
For this, 57 erenumab non-responders were switched to fremanezumab 225 mg 1× monthly sc. The interval to the last erenumab treatment was at least 2 months. Patients were 20-70 years old (mean age 49 years), 50 were women (87.7%), 7 were men. Patients had a mean of 19 headache days, 17 migraine days, and 13 triptan-taking days per month before the switch. 31 patients met criteria for medication overuse (54.3%) Mean MIDAS** was 117 (Fig. 2).
** Migraine Disability Assessment Score
Erenumab non-responders showed a 23% mean reduction in headache days per month, a 26% reduction in migraine days per month, and a 25% reduction in triptan days per month with fremanezumab 225 mg. The MIDAS score decreased by 22%. A 50% reduction in migraine days/month was achieved by 25% of patients. The 50% responder rate for headache was 21%, and a reduction in MIDAS score of at least 30% was achieved by 44%. Only one patient with a 50% reduction in headache days/month did not also have at least a 30% reduction in MIDAS score. 4% reported constipation and 1% injection reactions as side effects. Due to ineffectiveness, 44% discontinued further treatment. No patient discontinued treatment due to side effects. The mean duration of treatment up to the time of evaluation was 3.8 months.
The conclusion of the research team: Despite insufficient efficacy of a CGRP receptor antibody, there is still the possibility to achieve an effect in affected patients by switching to the CGRP ligand antibody fremanezumab. However, the probability of achieving at least a halving of headache days per month is lower (21%) than with the initial use of a CGRP receptor antibody. However, when the 30% MIDAS reduction is added as an additional efficacy criterion (as recommended in the German Migraine and Headache Society guidelines), 45.6% of erenumab non-responders can achieve significant clinical efficacy when switched to fremanezumab.
Congress: German Pain Congress 2020 (online)
Sources:
- Göbel C, et al: Immunotherapy with erenumab for episodic and chronic migraine: experiences in specialized pain therapy. German Pain Congress 2020 (online event), 21-24.10.2020, PO012.
- Göbel C, et al: Efficacy of fremanezumab in erenumab non-responders in the immunotherapy of migraine. German Pain Congress 2020 (online event), 21-24.10.2020, PO013.
InFo PAIN & GERIATRY 2021; 3(1): 19-20 (published 2/7/21, ahead of print).