Essential thrombocythemia usually occurs after the age of 50, although there are also those affected under the age of 40. Various subtypes can be distinguished based on molecular genetic analyses. Typical complications of the disease are thrombosis and bleeding. Risk stratification is necessary in order to provide patients with adequate treatment in good time. There are now various prognosis models that have been tested in large-scale cohort studies.
Myeloproliferative neoplasms (MPN) are diseases of the hematopoietic stem cells and exhibit a diverse spectrum of clinical manifestations and molecular abnormalities [1,2]. Essential thrombocythemia (ET) is an MPN that originates from a hematopoietic stem cell. A JAK2 mutation is found in around half of ET patients, less frequently there is a mutation in the CALR or MPL gene and in 10-15% of all ET patients none of these mutations are detectable. Although ET is considered the most indolent MPN, it is associated with distressing vasomotor symptoms and potentially fatal complications such as thrombosis, bleeding and disease progression to myelofibrosis and aggressive myeloid neoplasms [3]. Prognostic assessment of risk is important for timely risk-adapted intervention with available treatment options. However, risk prediction is difficult as it is a rare and clinically heterogeneous chronic disease [4].
Various prognostic models have been developed to assess the risk of thrombosis and overall survival (OS) in ET. Most prognostic systems to identify patients at risk of thrombosis and bleeding are based on the identification of risk factors such as thromboembolic complications or major bleeding, age ≥60 years, platelets ≥1.5 million/µl [5]. In the IPSET (International Prognostic Score for Thrombosis in Essential Thrombocythemia) or IPSET-Survival, cardiovascular risk factors and the presence of a JAK2 V617F mutation are also taken into account [6–8].
Patients with a CALR mutation show a lower risk of thrombosis compared to patients with a JAK2 mutation [9]. In the current “Triple-AAA” prognostic model for overall survival, age, absolute neutrophil count ( ANC) and absolute lymphocyte count ( ALC) are used for risk stratification in ET [10]. Even “non-driver mutations” can have an influence on the prognosis and are used, for example, in the MIPSS-ET (mutation-enhanced international prognostic system for essential thrombocythaemia) [11].
What are the most important risk factors?
Recently, two large retrospective ET cohorts were published by researchers from the US Mayo Clinic (Gangat et al.) and the Italian-based Center Research and Innovation of Myeloproliferative Neoplasms (Loscocco et al.) [12,13]. Both studies confirmed previously identified risk factors for thrombosis, progression and/or death in ET, which include the following:
- an older age (age ≥60),
- male gender,
- an increased white blood cell count (WBC >11 ×109/L),
- an increased absolute neutrophil count (ANC ≥8 ×109/L),
- a low absolute lymphocyte count (ALC <1.7 ×109/L) at the time of presentation.
Erdos et al. evaluated these parameters and current risk models in a cohort of 328 adult ET patients treated at Weill Cornell Medicine in New York (USA) over a median period of 6 years [14]. This cohort was strictly defined according to the World Health Organization diagnostic criteria of 2022, so that a diagnostic bone marrow biopsy was performed in all patients and alternative diagnoses were carefully excluded [15]. As Gangat et al. [12] and Loscocco et al. [13] Erdos et al. also found that patients with JAK2-mutated ET had a significantly shorter thrombosis-free survival (TFS) compared to patients with CALR-mutated ET. A total of 33 thromboses occurred (15 venous, 18 arterial), of which 27 (12 venous, 15 arterial) involved JAK2-mutated patients. In multivariable models, the increased risk of thrombosis in JAK2-mutated ET compared to CALR/MPL was primarily due to venous thrombotic events. Similar to CRIMM, IPSET was found to discriminate TFS primarily between very low and high risk ET, whereas TFS discriminated poorly between intermediate groups, with an area under the curve (AUC) of the receiver operating characteristic (ROC ) curve of only 0.63.
Conclusion of several independent studies
The study by Erdos et al. published in 2023 is consistent with the main findings of the two large cohorts by Gangat et al. and Loscocco et al. and confirms that the long-term risk of ET-related events can be stratified using readily available clinical information such as age, driver mutations and blood count [12–15].
Taken together, these independent studies prove the following:
- JAK2-mutated ET is associated with an increased risk of thrombosis compared to other ET types
- Thrombotic events rarely occur in patients with CALR-mutated ET
- CALR and MPL mutations significantly increase the risk of myelofibrosis progression
- the driver mutation is not prognostic for the OS
- the revised IPSET thrombosis score stratifies very low and high long-term thrombosis risk, but performs worse overall than the real data
- AAA and, to a lesser extent, IPSET survival are prognostic for overall survival (OS), with age having the strongest influence on risk.
In summary, these studies highlight the need for dynamic models of thrombosis, progression and survival in ET that include modifiable factors that can be targeted for risk reduction.
Primary therapeutic goal: risk-adapted thrombosis prophylaxis
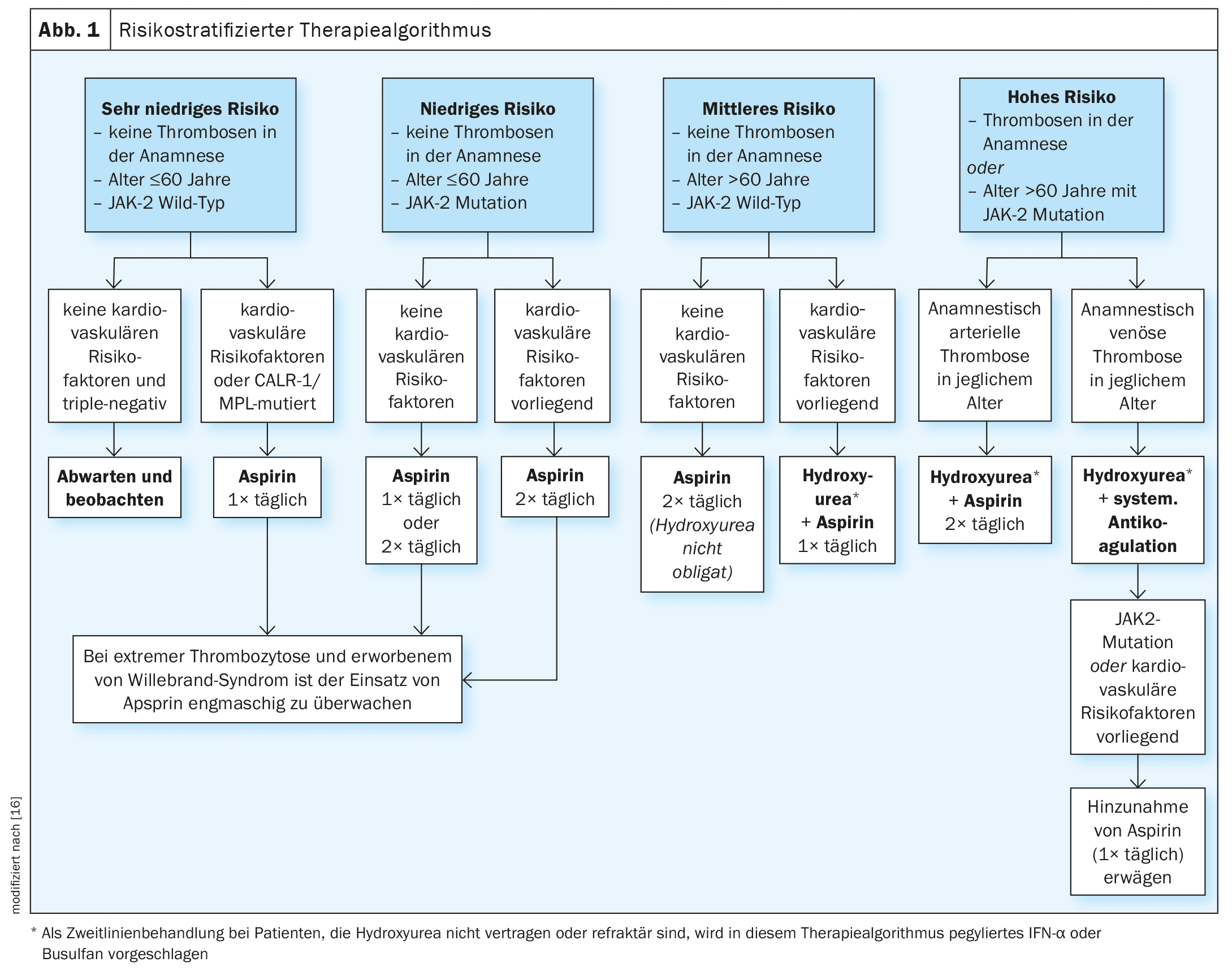
There is currently no curative therapy for ET. The primary treatment goals are to reduce disease-related symptoms and complications and to lower the risk of thromboembolism and cardiovascular risk factors. In a publication by Tefferi et al. published in 2024, a risk-stratified treatment strategy for ET is outlined (Fig. 1), with a classification into four risk levels [16].
- Very low (age ≤60 years, no history of thrombosis, JAK2 wild type)
- low (as very low, but JAK2 mutation present)
- Medium (age >60 years, no history of thrombosis, JAK2 wild type)
- high (history of thrombosis or age >60 years with JAK2 mutation).
The risk of thrombosis in patients with very low risk and triple negative mutation status is too low to justify any form of therapy. However, in the presence of cardiovascular risk factors or CALR/MPL mutations, the use of acetylsalicylic acid (aspirin, once daily) is recommended [12,13]. In low-risk patients, aspirin is recommended both in the absence and presence of cardiovascular risk factors. For patients at intermediate risk, aspirin twice daily is currently recommended, although the combination of a cytoreductive drug (hydroxyurea) with aspirin once daily has been shown to be effective in patients at intermediate risk with cardiovascular risk factors. These are evidence-based treatment recommendations. In the Mayo Clinic cohort, aspirin had a favorable effect on both arterial and venous thrombotic events, while similar protective effects were observed for cytoreductive therapy in the Florence cohort [12,13]. In patients with a bleeding tendency, the use of aspirin should be closely monitored or hematologically clarified.
Congress: EHA2024
Literature:
- Onkopedia: Myeloproliferative Neoplasias, www.onkopedia.com/de/onkopedia/guidelines/myeloproliferative-neoplasien-mpn-frueher-chronische-myeloproliferative-erkrankungen-cmpe/@@guideline/html/index.html, (last accessed 22.10.2024).
- Mahmud M, et al: Myeloproliferative Neoplasms: Contemporary Review and Molecular Landscape. Int J Mol Sci 2023; 24(24): 17383.
- Abu-Zeinah G, et al: Are thrombosis, progression, and survival in ET predictable? Blood Cancer J 2024; 14: 103, https://doi.org/10.1038/s41408-024-01079-7.
- Tefferi A, et al: Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014; 124: 2507-2513.
- “Essential thrombocythemia”, www.mll.com/myeloproliferative-neoplasien-mpn/essentielle-thrombozythaemie-et,(last accessed 10/22/2024)
- Barbui T, et al: Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 2012; 120(26): 5128-5133.
- Haider M, et al: Validation of the revised International Prognostic Score of Thrombosis for Essential Thrombocythemia (IPSET-thrombosis) in 585 Mayo Clinic patients. Am J Hematol 2016; 91(4): 390-394.
- Barbui T, et al: Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 2015; 5: e369-e36.
- Torregrosa JM et al: Impaired leucocyte activation is underlining the lower thrombotic risk of essential thrombocythaemia patients with CALR mutations as compared with those with the JAK2 mutation. Br J Haematol 2016; 172(5): 813-815.
- Tefferi A et al: Globally applicable “triple A” risk model for essential thrombocythemia based on Age, Absolute neutrophil count, and Absolute lymphocyte count. Am J Hematol 2023; 98(12): 1829-1837.
- Tefferi A, et al: Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br J Haematol 2020; 189: 291-302.
- Gangat N, et al: One thousand patients with essential thrombocythemia: the Mayo Clinic experience. Blood Cancer J 2024; 14: 1-11.
- Loscocco GG, et al: One thousand patients with essential thrombocythemia: the Florence-CRIMM experience. Blood Cancer J 2024; 14: 1-12.
- 14 Erdos K, et al: Low thrombosis risk CALR mutations confer higher risk of essential thrombocythemia progression. Blood 2023; 142: 1819-1819.
- Abu-Zeinah G, et al: Interferon-alpha for treating polycythemia vera yields improved myelofibrosis-free and overall survival. Leukemia 2021; 35: 2592-2601.
- Tefferi A, Vannucchi AM, Barbui T: Essential thrombocythemia: 2024 update on diagnosis, risk stratification, and management. Am J Hematol 2024; 99(4): 697-718.
HAUSARZT PRAXIS 2024; 19(11): 32-33 (published on 22.11.24, ahead of print)