If a diagnosis of severe asthma is confirmed, adjunctive therapy with monoclonal antibodies may be considered. Currently, anti-IgE or anti-IL5 antibodies are available for this purpose, depending on the phenotype. Consensus recommendations advise follow-up and adjustment of the treatment regimen if necessary.
Using a case example, Prof. Dr. Christian Taube, Director of Pneumology at the University Hospital Essen (D), illustrated the relevance of diffusion capacity as a differential diagnostic parameter, among other things, at DGIM 2019 [1]. The 55-year-old patient, who never smoked, experienced repeated dyspnea on exertion. Previous treatment was with inhaled steroid in fixed combination with long-acting beta-2 sympathomimetic. In the course, acute exacerbations of the symptoms occurred repeatedly, which made treatment with systemic steroids necessary to achieve a significant improvement. A body plethysmography revealed the following findings: obstructive airway disease, severe obstruction, and pulmonary hyperinflation. The following further diagnostic clarifications were performed: Diffusion capacity, X-ray chest, measurement of nitric oxide in exhaled air, allergy testing. “In COPD, there is marked emphysema in most patients and thus diffusion capacity is also reduced – this is one of the most sensitive functional parameters,” the speaker explained. In addition, to rule out carcinoid, pulmonary imaging should always be performed in such complaints. In the patient in the case study, the following diagnosis could be made by this diagnostic procedure: severe bronchial asthma; recurrent exacerbations more than three times a year; no sensitization detectable; no ASA (acetylsalicylic acid) intolerance.

Eosinophil count decisive for indication
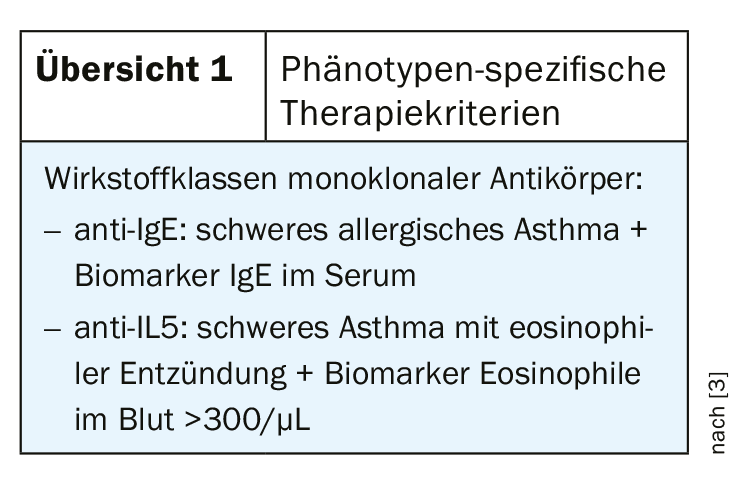
In the presence of a confirmed diagnosis of severe asthma, consideration should be given to whether treatment with monoclonal antibodies should be considered. At present, the following two classes of active ingredients are approved (overview 1):
- anti-IgE (omalizumab) for the indication of severe persistent allergic bronchial asthma (exacerbation, marked obstruction, and sensitization to year-round allergen, e.g., dust mites or Aspergillus)
- anti-IL5 (mepolizumab, reslizumab) for the indication of severe eosinophilic asthma (eosinophilic inflammation). For the detection of eosinophilic inflammation, differential blood counts are an economical and feasible method, and it is not the percentage of eosinophils but the absolute number of eosinophilic granulocytes that is important in this context. Eosinophilia in the blood is a well established biomarker and increased eosinophil count correlates with higher frequency of severe exacerbations, poorer disease controllability and better response to anti-eosinophil therapy [2].
Response-dependent long-term therapy possible
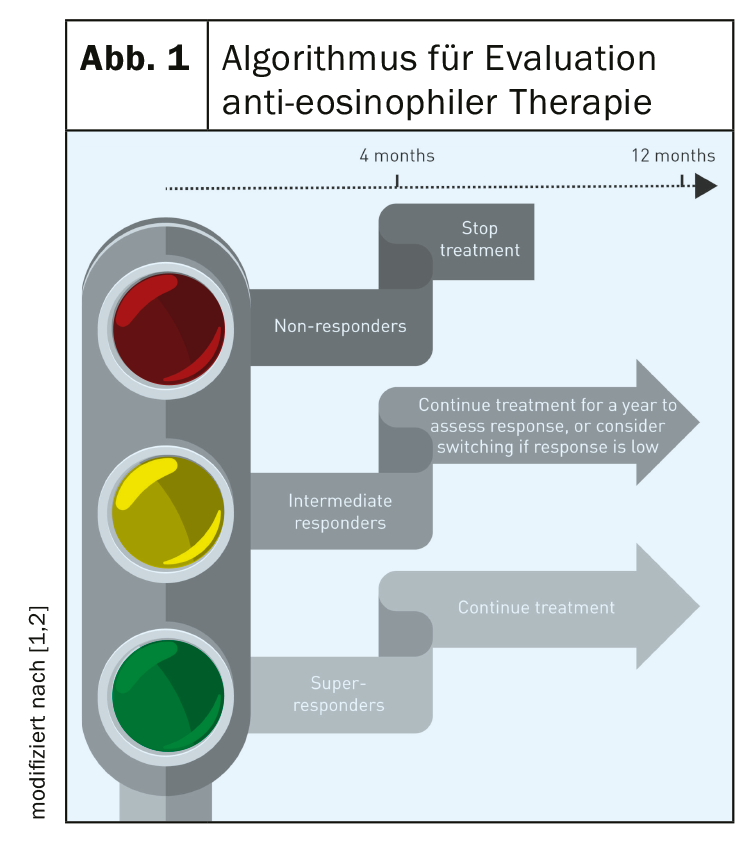
According to Buhl et al. [2], further evaluation studies are needed on the efficacy of antibody therapy in different subpopulations of patients with severe eosinophilic asthma. These are non-curative adjunctive therapies, so criteria for changing or discontinuing therapy are important. The European expert task force has worked out the following algorithm (Fig. 1): Evaluation of the response after a minimum therapy duration of 4 months – in “super-responders” therapy can be continued; “Intermediate-responders” should continue anti-IL5 antibody therapy for 1 year and then reassess response or switch to another antibody therapy; “non-responders” should discontinue anti-eosinophil therapy. The question of whether anti-IL5 antibody therapy may lead to decreased use or even elimination of oral corticosteroids in the future cannot be answered at this time. In contrast, the fact that patients with severe asthma experience symptoms associated with systemic corticosteroid therapy significantly more often than those with moderate severity and non-asthmatics was empirically demonstrated in a large-scale British study (93% vs. 78% vs. 64%; p<0.001) [5]. In severe asthma, morbidity rates regarding symptoms associated with systemic corticosteroid therapy were significantly higher compared with a moderate form of asthma (type 2 diabetes 10% vs. 7%, OR=1.46; 95% CI: 1.11-1.91; p<0.01; osteoporosis 16% vs. 4%, OR=5.23; 95% CI: 3.97-6.89; p<0.001; dyspeptic disorders (including gastric/duodenal ulceration) 65% vs. 34%, OR=3.99; 95% CI: 3.37-4.72; p<0.001; cataracts 9% vs. 5%, OR=1.89; 95% CI: 1.39-2.56; p<0,001) [5].
anti-IL-5 antibodies an option for EGPA in the future?
The fact that treatment with anti-IL5 antibodies could also represent a therapeutic option in the future for the treatment of eosinophilic granulomatosis with polyangiitis (EGPA; formerly known as Churg-Strauss syndrome) was demonstrated by Prof. Dr. Dr. med. Claus Kroegel, head of the functional area of pneumology at Jena University Hospital, on the basis of a case study (box Case study) [6].

The 55-year-old female patient with severe allergic cortisone-dependent bronchial asthma with polyvalent allergic sensitization developed recurrent paresthesias in both soles of the feet and persistent pain in the toes and fingertips ten months after cessation of therapy with omalizumab in October 2015. Subsequently, treatment with 10 mg MTX per week s.c. was initiated. During the course, the patient should slowly reduce the cortisone dose from 10 mg/day (7.5 mg for 2 months, then 5 mg for 2 months, then further reduce in 1 mg increments). At follow-up 4 months later, the patient reported a slight improvement in dyspnea and cough. However, the paresthesias were progressive and now continuously present (forefeet, soles, toes, fingers). Reduction of oral cortisone dose below 7.5 mg/day had not been possible. In addition, two exacerbations occurred, each requiring cortisone pulses. In February 2017, treatment with 10 mg MTX per week s.c. was discontinued and treatment with the anti-IL-5 antibody mepolizumab (100 mg per month) was initiated; cortisone therapy was to be continued. At follow-up 6 months later, the patient reported significant health improvement. Dyspnea and coughing are now rare, she feels physically fit again and can breathe freely. The rhinosinupathy had disappeared and the paresthesias in the area of the soles of the feet had decreased. She reduced the cortisone dose to 5 mg, she said, and cortisone pulses were no longer needed. According to her own information, the patient is doing excellently as a result.
The diagnosis of eosinophil-associated disease, such as EGPA, is often complicated by the need for oral corticosteroids, the speaker explained. The manifestation of the typical symptoms, in particular vasculitis, pulmonary changes and eosinophilia, is suppressed by this. In this case, extrapulmonary manifestations (paresthesias as vasculitis equivalent), elevation of IgG4, and finally response to anti-eosinophil therapy had led to the diagnosis. IgG4 can be detected in half of the patients with EGPA, but the significance of this immunoglobulin in this context is unclear at this time.
Literature:
- DGIM: Prof. Dr. Christian Taube, Director of Pneumology at the University Hospital Essen, slide presentation: Eosinophils: 125. Congress DGIM, Wiesbaden, 4. May 2019.
- Buhl R, et al: Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J 2017; 49: 1700634.
- Ortega H, et al: Blood eosinophil counts predict treatment response in patients with severe eosinophilic asthma. J Allergy Clin Immunol 2015; 136 (3): 825-826.
- Taube C, et al: Bronchial asthma: is personalized therapy on the horizon? Allergo J Int 2014; 23(7): 246-251.
- Sweeney J, et al: Thorax 2016; 71(4): 339-346.
- DGIM: Prof. Dr. Dr. med. Claus Kroegel, Head of the Functional Area of Pneumology at the University Hospital Jena, slide presentation: Eosinophils: 125. Congress DGIM, Wiesbaden, May 4, 2019.
InFo PNEUMOLOGY & ALLERGOLOGY 2019; 2(1): 21-22.