Epithelial ovarian carcinoma is a heterogeneous group of carcinomas that have different etiology, genetics, and thus biological prognosis. In advanced stages (IIB-IV), the standard therapy remains maximal cytoreductive therapy, weighing morbidity and prompt continuation of chemotherapy with carboplatin/paclitaxel. Adjuvant chemotherapy remains carboplatin and paclitaxel (q3w or q1w). In R1 situation or stage IV, additional bevacizumab should be administered during therapy and continued as maintenance therapy, as this showed a survival benefit of approximately nine months. Individualization of therapy according to the underlying biology is essential, therefore the involvement of all experts and an interprofessional and interdisciplinary team in a tumor center is needed.
Approximately 200,000 women worldwide develop epithelial ovarian cancer (EOC). Approximately 60-70% of patients are diagnosed at an advanced stage (FIGO stage III-IV). Of these patients, approximately 70% experience a recurrence, which is usually a controllable but no longer curative situation. Prognosis in the advanced stages is modest with 50% 5-year survival. In the early stages (FIGO I-IIA), patients are usually cured after adequate surgical staging and adjuvant platinum-containing systemic therapy (ACTION trial). However, age-standardized European incidence and mortality rates have changed only slightly over the past 40 years despite improved surgical subspecialization and new second-line drugs.
Symptomatology
The symptoms of EOC are usually nonspecific and appear late. The commonly complained symptoms are often of gastrointestinal origin: meteorism, changing bowel habits, micturition difficulties, and increase in abdominal girth. The clinically most important sign is a solid, irregular, fixed tumor in the lesser pelvis that can be delineated on gynecologic examination.
Histological subtypes
The histologic subtypes of ovarian cancer are well delineated morphologically: serous (75-80%), endometrioid (10%), mucinous (10%), clear cell (1%), transsitional cell (1%), and undifferentiated (1%). Not only do these histotypes differ purely morphologically, they also show a different biological behavioral pattern, which is represented, among other things, by a clear genetic distinction at the mRNA level [1]. And the clinical course also differs depending on the biological subtypes. Thus, the prognosis of endometrioid EOC is much better than that of mucinous EOC [2]. In the future, therapy will have to be increasingly oriented towards biological subtypes in order to achieve better prognoses.
For a long time, ovarian cancer was thought to occur most sporadically and to be hereditary in only 10-15% of cases. Hereditary mutations relevant to ovarian cancer are in the BRCA1 (Chr. 17.60%) and BRCA2 (Chr. 13.30%) genes, as well as mutations in the DNA mismatch-repair genes MLH1, MSH2, MSH6, PMS1, PMS2 (5%), which also cause “human non polyposis colon cancer” (HNPCC) or Lynch syndrome [3]. A recent Australian study showed that as many as 20% of all serous carcinomas have a mutation in the BRCA1 or -2 gene [4]. In undifferentiated serous carcinomas, we know that there is a so-called “BRCAness” phenotype. Since both BRCA and PARP play a role in tumor resistance modification, treatment with so-called PARP inhibitors is central, and this is likely to increase in the future.
Our knowledge of the development of ovarian cancer was revolutionized in 2007 by having the adnexa removed from BRCA1/2 mutation carriers and discovering early in situ tubal carcinomas here. This led to the realization that a significant proportion of serous ovarian carcinomas probably originate from the tube. Previously, the single-layered surface epithelium covering the ovary had been thought to be altered by continuous ovulation, producing metaplastic-altered inclusion cysts [5, 6].
Therapy of ovarian cancer
Therapy for ovarian cancer is multimodal and includes surgery and chemotherapy or target therapy and, in the past, radiotherapy.
As early as the 1940s, it was first postulated that complete resection of all tumor remnants was essential for prognosis. However, it was only with the subspecialization of special gynecologists as gynecologic oncologists that it became possible to perform debulking surgery below 2 cm. Here, complete rather than optimal cytoreduction should be pursued whenever possible [7, 8]. Optimal debulking is a residual tumor focus <1 cm (achievable in 47%) according to the 2010 old GCIG (Gynecological Cancer Intergroup) Consensus [9]. In the new GCIG consensus definition, however, optimal debulking corresponds to no macroscopically visible residual tumor (achievable in only 34%) [10].
Several studies have shown that survival is significantly dependent on residual tumor volume. A 2002 meta-analysis of over 6000 patients by Bristow clearly demonstrated the linear relationship of extent of cytoreduction and
Survival at [8]. Current topics of discussion concern the extent of aggressiveness in debulking, e.g., upper abdomen [11] or lymph nodes. In the study of a large US tumor center, it was shown that complete cytoreduction was possible in 85.3% and optimal cytoreduction (<1 cm) in another 13.5%. However, this was only possible with massively increased morbidity due to extensive additional interventions, e.g. rectosigmoid resections (52.1%), diaphragm stripping (40.5%), ablation of peritoneal implants (89%) or also splenectomies, liver resections as well as distal pancreatectomies (19%) [7]. Since therapy of EOC involves not only surgical debulking but also prompt continuation of therapy with chemotherapy, it seems questionable whether extended debulking with high morbidity and the delay of subsequent chemotherapy by several months is useful, especially in patients of advanced age.
At the current time, there are no studies that have shown a benefit of lymphonodectomy. This is especially true in the case of a residual tumor regardless of volume. A retrospective analysis of study patients from various AGO-OVAR trials (n=3388) showed a small benefit in terms of overall survival in the 51.3% of optimally tumor-reduced patients when systematic pelvic/paraaortic lymphonodectomy was performed [12]. Apart from this one retrospective observation, however, there are no prospective data to prove the factual point.
Surgical therapy
Surgical therapy of EOC should always be performed by longitudinal laparatomy if malignancy is urgently suspected. Laparascopy has no value in primary therapy of EOC and increases the risk of tumor or cyst rupture. If intraoperative iatrogenic tumor rupture occurs, the prognosis worsens with “upstaging” to a stage IC. Tumor treatment should always include complete staging at early stages and debulking at advanced stages, as this is also prognostic. Compromises can be made in fertility-preserving surgery with unilateral adenexectomy. However, in the case of incomplete staging, “upstaging” occurs in 30% of cases during the second operation. Table 1 shows the requirements for surgical therapy including fertility-preserving surgery in early staging. Because advanced mucinous carcinomas are usually of non-ovarian origin, appendectomy and extensive exploration of the bowel/stomach should always be performed as well [13].

In contrast to the treatment of early EOC, the treatment of advanced EOC is designed to maximize cytoreductive surgery. After FIGO stage, tumor residual after maximal resection is the second most important prognostic
Factor. All visible tumor lesions should be removed. Here, complete resection is much better than resection <1 cm (1-10 mm). An AGO meta-analysis showed that complete resection prolonged survival by 30 months in stage IV, 47 months in stage IIIC, and 60 months in stage IIB-IIIB [14].
Bowel, diaphragmatic, and partial hepatic resection may also improve outcome, but they also impose corresponding morbidity and mortality and should be performed only by experienced gynecologic oncologists.
Radiotherapy
In the 1980s, radiotherapy was used as adjuvant therapy because it showed a benefit over chemotherapy with cyclophosphamides. However, it has been relegated to the background nowadays by the introduction of platinum-containing chemotherapy and, despite its good efficacy, is used only rarely. However, in the palliative situation, radiotherapy for symptom control still has an important role and it may be considered even after optimal secondary cytoreduction in localized recurrence.
Chemotherapy
Today, platinum-containing chemotherapy is the standard of care in adjuvant treatment of ovarian cancer as well as in recurrence with an appropriate interval (>6 months). In the 1980s, various combination therapies were evaluated, particularly the combination of platinum with cyclophosphamide or doxorubicin. Triple combinations were also studied, but overall produced more toxicity than benefit to patients.
Taxanes, initially derived from the Pacific yew tree (Taxus brevifolia), represent the second mainstay of chemotherapy. The combination of carboplatin and paclitaxel remains the standard of care in adjuvant chemotherapy of ovarian cancer and has been reviewed in various trials (GOG111, OV10, ICON3). Since 2004 , the combination of carboplatin/paclitaxel i.v. has been the worldwide standard based on trial data. This was also recommended in a consensus paper [9].
Several studies have examined the use of chemotherapy in low-risk versus high-risk groups (GOG, ICON 1, ACTION). Current discussions revolve around the administration of neoadjuvant chemotherapy (EORTC55971,
CHORUS trial), intraperitoneal chemotherapy (SWOG, GOG172), to weekly administration of paclitaxel (JGOG3016, MITO7, ICON8), additional administration of target therapies, especially bevacizumab (ICON7, GOG218, AURELIA, OCEANS), as well as to ongoing so-called “postchemotherapy”. “Maintenance” – that is, maintenance chemotherapy with bevacizumab.
The phase III trials that have heralded a paradigm shift in standard therapy in advanced ovarian cancer over the decades were:
- GOG111 (n=386) and OV10 (n=680), which compared cisplatin/cyclophosphamide with cisplatin/paclitaxel and showed a survival benefit for the paclitaxel combination
- GOG158 (n=792) and OVAR3 (n=883), which compared cisplatin/paclitaxel with carboplatin/paclitaxel and showed similar efficacy with better tolerability for the carboplatin
- GOG172 (n=429), which compared iv administration of cisplatin/paclitaxel with intraperitoneal administration and observed improved efficacy but increased toxicity and decreased quality of life in patients
- JGOG3016 (n=637), which compared the carboplatin/paclitaxel combination as a weekly versus a three-weekly taxol regimen and showed a survival benefit for weekly paclitaxel therapy
- GOG218 (n=1873) and ICON7 (n=1528), which evaluated carboplatin/paclitaxel with placebo versus carboplatin/paclitaxel with and without continued administration of bevacizumab and showed a significant survival benefit for patients who had advanced carcinoma (FIGO III/IV) and could not undergo optimal surgery (ICON7).
Bevacizumab is the first drug in the list of listed therapies that has been developed as a new “targeted therapy” in the context of genetic testing in ovarian cancer. It attacks tumor vessels by binding to vascular endothelial growth factor (VEGF), which in turn binds to the receptor of the same name. VEGF is responsible for endothelial cell survival, vascular abnormalities, stimulation of new vessel growth, and enhanced vascular permeability [15]. He was one of the top candidates in the analysis of dysregulated genes in ovarian cancer [16]. VEGF plays a central role in normal ovulation, VEGF-stimulated angiogenesis is essential for tumor growth, and plays an important role in ovarian cancer development by promoting changes from benign to malignant growth and contributing to the formation of peritoneal metastases and ascites production [17]. It is therefore not surprising that high levels of VEGF in the blood are associated with a poorer prognosis. There are now four phase III trials of the VEGF inhibitor bevacizumab in ovarian cancer, both adjuvant (GOG-0218 and ICON7 [18, 19]) and first-line palliative in platinum-sensitive and -refractory settings (OCEANS and AURELIA [20, Pujade-Lauraine ASCO 2012]).
Treatment of early-stage (FIGO IA G1-FIGO IIA) ovarian cancer includes platinum-containing chemotherapy; this improves survival prognosis by about 8% at five years. Stage IA G1 tumors should not receive chemotherapy because they have an excellent prognosis with surgery alone. All other early-stage patients receive chemotherapy with carboplatin/paclitaxel every three weeks for a total of four to six cycles.
In carboplatin/paclitaxel combination therapy, paclitaxel may optionally be administered “dose-dense” weekly. The Japanese data (JGOG3016) are excellent, but it is still unclear to what extent the data are transferable to European patients, although it was the serous carcinomas rather than the clear cell carcinomas that showed the response. The European MITO7 trial showed no PFS (progression-free survival) benefit with weekly administration of carboplatin and paclitaxel, although better tolerability.
Data from the ICON8 trial, which is also testing the dose-dense approach in one arm, are now eagerly awaited.
Recurrence treatment
Approximately 70% of patients diagnosed with advanced stage FIGO III/IV will relapse during the first five years after adjuvant therapy. Then, unfortunately, there is a palliative situation with usually no chance of cure. However, in contrast to the past decades, the recurrence situation is characterized by the fact that therapeutic options have increased and stable recurrence can often be achieved for years.
The goals of relapse therapy must include symptom control, prolongation of symptom-free survival, and maintenance/improvement of quality of life. The management of these patients is becoming more complex as data accumulate, and the choice of which treatment to use or to wait cautiously depends on individual factors. However, treatment decisions should be evidence-based whenever possible and should come from randomized trials. Repeat surgical intervention is only useful for selected patients, i.e. if, for example.
- The patient is recurrence-free for more than two years
- The tumor seems completely resectable
- An isolated lymph node involvement is present
- No ascites or peritoneal carcinomatosis present
- The patient shows a good perfomance.
Prospective data on re-resection are currently being generated in the DESKTOP-III trial. This study randomly reviews the value of re-resection for platinum-sensitive recurrence. Experience has already been gained in the DESKTOP-II study and a predictive score for operability has been developed [21].
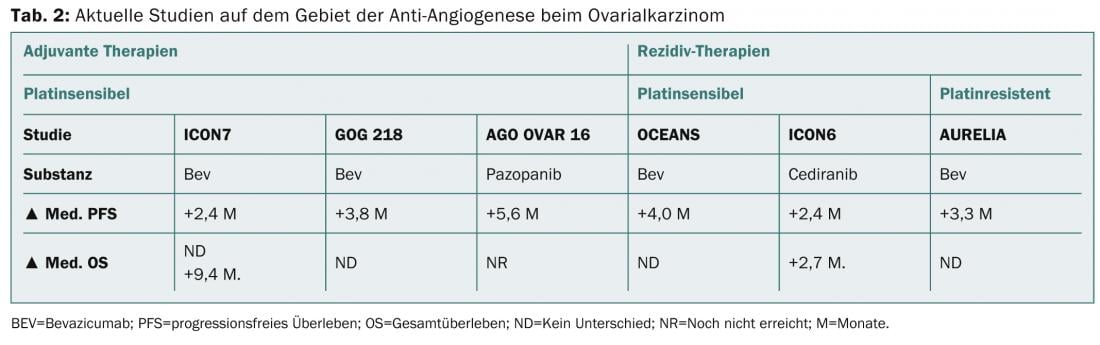
New chemotherapy options for relapsed ovarian cancer in the platinum-sensitive as well as -refractory situation may be the additional administration of bevacizumab or also the weekly administration of paclitaxel. Table 2 shows current developments in the field of antiangiogenesis in initial therapy as well as in recurrence therapy.
In therapy-refractory patients, good palliative care is also needed, which has already been included in the therapy decisions in advance. The prognosis in this situation is usually very poor (<1 year survival) and symptom control is thus the primary concern.
In general, the following cofactors should be carefully considered when choosing a palliative chemotherapeutic agent: Time to relapse, adjuvant chemotherapeutic agents received, current symptoms, patient wishes and comfort (alopecia?), general condition, comorbidities, tolerance of prior chemotherapy, residual toxicity (polyneuropathy), and organ function. If the ECOG status of patients with platinum-sensitive tumors is good, a platinum-containing combination with a taxane/anthracycline is indicated because of improved prognosis compared with singular platinum therapy [22]. Inclusion of these patients in a possible clinical trial is generally recommended.
Due to the increasing complexity, an ovarian cancer patient should always be treated in an interdisciplinary interprofessional team within the framework of a gynecologic tumor center. This has the advantage not only of greater experience with these patients, but also in particular of the close networking of the individual disciplines and the optimal coordination of supportive and therapeutic measures.
Marcus Vetter, MD
Prof. Dr. med. Viola Heinzelmann-Schwarz
Literature:
- Heinzelmann-Schwarz VA, et al: Br J Cancer 2006; 94(6): 904-913.
- Winter III WE, et al: JCO August 20 2007; 25(24): 3621-3627.
- Burke W, et al: JAMA 1997; 277(12): 997-1003.
- Alsop K, et al: J Clin Oncol 2012; 30(21): 2654-2663.
- Auersperg N, et al: Endocr Rev 2001; 22(2): 255-288.
- Jarboe E, et al: Int J Gynecol Pathol 2008; 27(1): 1-9.
- Eisenkop SM, Friedman RL, Wang HJ: Gynecol Oncol 1998; 69(2): 103-108.
- Bristow RE, et al: Gynecol Oncol 2002; 86(2): 163-170.
- du Bois A, et al. (Gynecologic Cancer Intergroup; AGO-OVAR; ANZGOG; EORTC; GEICO; GINECO; GOG; JGOG; MRC/NCRI; NCIC-CTG; NCI-US; NSGO; RTOG; SGCTG; IGCS; Organizational team of the two prior International OCCC): Ann Oncol 2005; 16(8): viii7-viii12.
- Stuart GC, et al. (Participants of4th Ovarian Cancer Consensus Conference (OCCC); Gynecologic Cancer Intergroup): Int J Gynecol Cancer 2011; 21(4): 750-755.
- Barlin JN, et al: Gynecol Oncol 2013; 130(2): 284-288.
- du Bois A, et al. (Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens. Potential role of lymphadenectomy in advanced ovarian cancer): J Clin Oncol 2010; 28(10): 1733-1739.
- Schilder JM, et al: Gynecol Oncol 2002; 87(1): 1-7.
- du Bois A, et al: Cancer 2009 Mar 15; 115(6): 1234-1244.
- Ferrara N: Endocr Rev 2004; 25(4): 581-611.
- Jacob F, et al: Biomark Med 2009; 3(6): 743-756.
- Belotti D, et al: Cancer Res 2003; 63(17): 5224-5229.
- Burger RA, et al. (Gynecologic Oncology Group): N Engl J Med 2011; 365(26): 2473-2483.
- Perren TJ, et al. (ICON7 Investigators): N Engl J Med 2011; 365(26): 2484-2496.
- Aghajanian C, et al: J Clin Oncol 2012; 30(17): 2039-2045.
- Harter P, et al: Int J Gynecol Cancer 2011 Feb; 21(2): 289-295.
- Raja FA, et al: Ann Oncol 2013 Dec; 24(12): 3028-3034.
InFo Oncology & Hematology 2014; (2)1: 8-13.











