The link between diabetes and tuberculosis (TB) is increasingly becoming a global health concern as diabetes becomes more prevalent in regions where TB is endemic and immunodeficiency in diabetic patients increases. American physicians have described a rare case of extrapulmonary tuberculosis (EPTB) in a patient with type 1 diabetes mellitus and dermatomyositis on immunosuppressive therapy.
The case highlights important diagnostic considerations in certain patient groups and raises several interesting questions about the pathophysiology underlying this patient’s complex disease presentation: A 22-year-old man with uncontrolled type 1 diabetes mellitus, an HbA1c of 11.8%, and dermatomyositis (NXP2+) presented to Dr. Caroline Jansen’s team at Emory University School of Medicine in Atlanta, Georgia [1]. The patient had had a fever (max. 39.4 °C) for 3 days, severe pain in the left thigh and was unable to walk. Treatment for dermatomyositis included prednisone, methotrexate, tofacitinib, and intravenous immunoglobulin during relapses. He was prescribed 25 U insulin glargine twice daily and 10 to 12 U insulin lispro with meals. He had a history of a left lower extremity (LLE) abscess that had been incised and drained 2 years previously, in which group B Streptococcus and methicillin-susceptible Staphylococcus aureus were proliferating. He was diagnosed with pneumonia four months ago and was treated with levofloxacin/linezolid as per experience. On arrival, he reported that he had no pulmonary symptoms and had not used drugs or been sexually active in the past year. He had recently worked in a funeral parlor.
On arrival, the patient was tachycardic, normotensive and afebrile. Physical examination revealed a painful, warm and reddened left knee and distal lateral thigh with limited range of motion due to pain, point-of-care ultrasound showed no fluid collection over the left distal lateral thigh. Laboratory results were notable for hyperosmolar hyponatremia (122 mmol/l), hyperglycemia (670 mg/dl) with elevated beta-hydroxybutyrate, metabolic acidosis, and thrombocytosis (648×109/l). Further tests revealed an increased D-dimer value, C-reactive protein (50 mg/dl) and an increased erythrocyte sedimentation rate (94 mm/h).
Detection of a M. tuberculosis infection
The infectious disease examination revealed a negative COVID-19 polymerase chain reaction test, a positive (1.3) beta-D-glucan value and a positive QuantiFERON-Gold for the detection of a Mycobacterium tuberculosis infection. Computed tomography of the chest showed a cavitary lesion in the left lower lobe. In addition, bronchoalveolar lavage revealed acid-fast bacilli, and the corresponding culture was positive for M. tuberculosis. In addition, magnetic resonance imaging (MRI) of the left lung showed inflammatory myositis/fasciitis. MRI of the spine showed chronic changes consistent with chronic steroid treatment, with no evidence of Pott’s disease.
On admission, vancomycin + piperacillin/tazobactam, intravenous immunoglobulin (2 g/kg), insulin and increased doses of prednisone were administered. As there were no risk factors for drug-resistant disease, rifampicin, isoniazid, pyrazinamide and ethambutol (RIPE) were administered after confirmation of tuberculosis and a negative GeneXpert MTB/RIF test. After a moderate improvement in symptoms was observed following the start of treatment, the patient was discharged home with the recommendation of further follow-up examinations.
HbA1c after 3 months at 13.0%
Three months later, the patient presented again complaining of several weeks of increasing back and LLE pain accompanied by facial swelling, fever, malaise and anorexia. He did not report night sweats, cough, sputum production, skin lesions, muscle weakness or dysuria. He was also adherent to TB treatment (rifampicin/isoniazid) and his dermatomyositis treatment regimen, was afebrile and tachycardic. Examination findings revealed diffuse tenderness of the bilateral lower extremities with a sensitive fluctuant area in the center of the left posterolateral thigh.
The laboratory tests revealed hyperglycemia (679 mg/dl), an HbA1c value of 13.0%, a C-reactive protein of 9.9 mg/dl, a erythrocyte sedimentation rate of 93 mm/h and an aldolase of 14.6 U/dl.
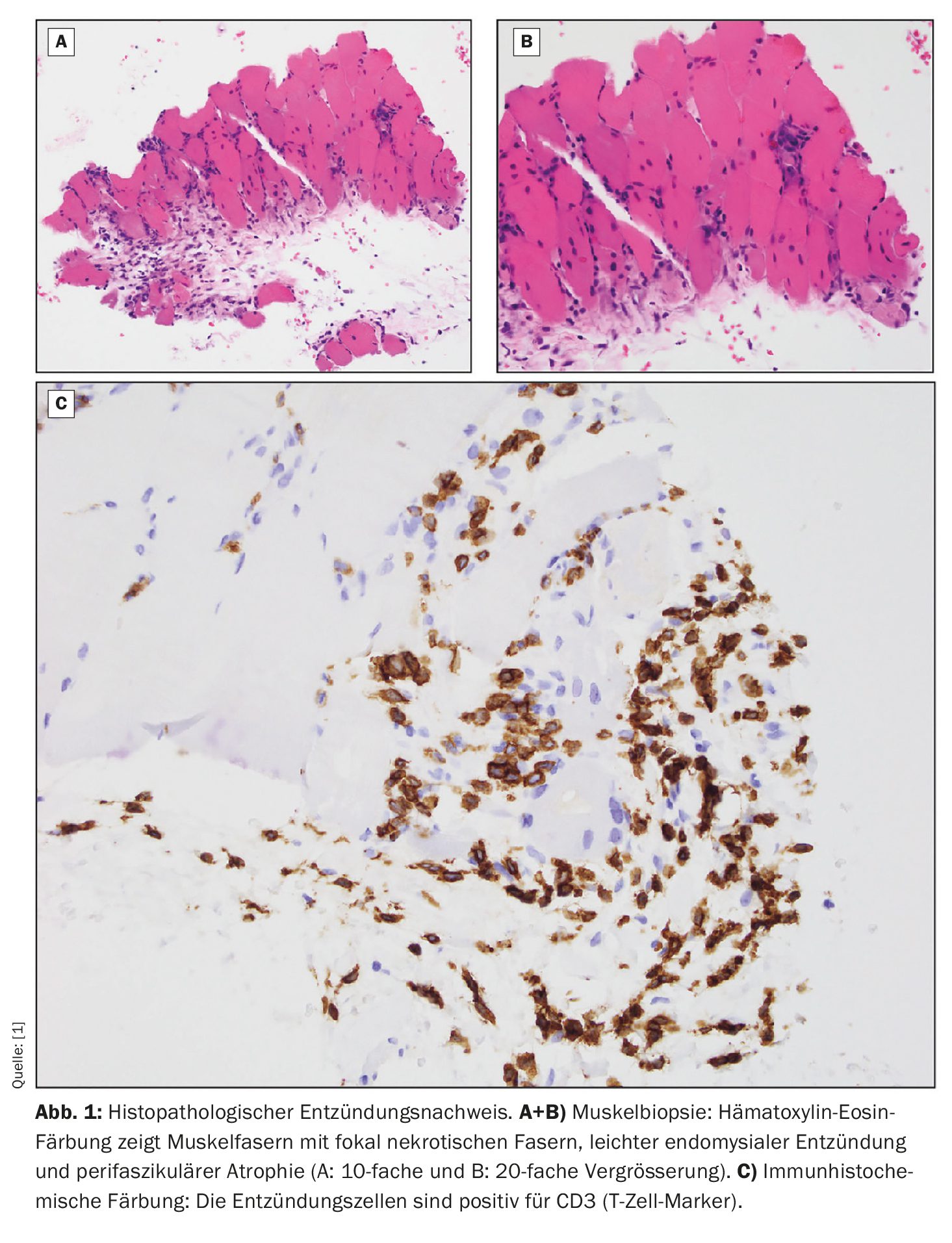
In addition, MRI of the lower extremities showed bilateral diffuse intramuscular and myofascial edema and a complex, multilocular, intermuscular, subfascial fluid collection in the proximal-to-midposterior compartment of the left thigh. According to Dr. Jansen, aspiration of the fluid collection was preferred over surgical drainage. Histopathology revealed focal necrotic muscle fibers and perifascicular atrophic fibers with mild endomysial inflammation (Fig. 1). Tissue staining was positive for CD3 and CD20. The anaerobic, aerobic and fungal cultures from the aspirate were negative. Culture to confirm acid-fast bacilli revealed M. tuberculosis , with tests showing pan-sensitivity to RIPE.
Higher risk of tuberculosis in diabetes patients
This unusual case of EPTB with type 1 diabetes mellitus demonstrated a complex presentation of TB myositis in an immunocompromised patient and highlighted the intricate interplay between risk factors and disease development, the complexity of immune mechanisms behind EPTB, and the variability of clinical presentations of pulmonary and extrapulmonary TB.
A definitive diagnosis often requires histopathologic, cultural and molecular testing. According to the authors, it is often delayed due to the extensive differential diagnosis for myositis-like symptoms. The patient’s condition did not improve with therapy for an episode of dermatomyositis, and symptomatic improvement was not observed until RIPE therapy was initiated. At his subsequent presentation, a dermatomyositis flare was less likely as the patient’s immunosuppressive therapy had been tapered since his TB diagnosis and he had no muscle weakness. Given the improvement in his symptoms after starting RIPE therapy, the primary diagnosis was TB-related myositis. Immunosuppression is a known risk for TB, and the risk of TB is particularly high in immunocompromised patients, such as HIV patients or patients taking disease-modifying antirheumatic drugs.
The link between diabetes and TB is a major health challenge given the increasing prevalence of diabetes in TB endemic areas and the recognition of immunodeficiency in diabetic patients. Diabetes is associated with a higher risk of TB and unfavorable treatment outcomes. The mechanism driving this is unknown, although impaired cytokine production may contribute, the authors emphasize. Dr. Jansen and colleagues note that their patient’s HbA1c worsened between presentations, which could be due to an increase in his steroid therapy or continued difficulty accessing his medications. In either case, his diabetes may have contributed to his infection, emphasizing the importance of access to critical medications.
This 22-year-old man with type 1 diabetes presented with symptomatic EPTB, with no pulmonary symptoms despite a cavitary lung lesion. Immunocompromised patients, such as those with HIV/TB co-infection, are known to present with atypical symptoms, which may partially explain the patient’s presentation.
Months prior to presentation, the patient was diagnosed with left lower lobe pneumonia and an exudative parapneumonic effusion and was treated empirically with levofloxacin/linezolid. Although cultures were not obtained, this pneumonia likely represented the developing TB cavity. According to the authors, it may be advisable to opt for definitive culture-guided treatment, especially in immunocompromised patients, as they are at increased risk of TB infection.
Literature:
- Jansen C, et al.: Mycobacterium tuberculosis Myositis Without Concurrent Pulmonary Symptoms in a Patient With Immunosuppression. AIM Clinical Cases 2024; 3: e230950; doi: 10.7326/aimcc.2023.0950.
InFo DIABETOLOGIE & ENDOKRINOLOGIE 2024; 1(4): 28–29