Narrowing of the aortic valve is the most common acquired valve defect and is corrected by AKE or TAVI. However, despite advances in therapy, rhythm problems often occur. What to do?
In Switzerland, thousands of patients undergo surgical aortic valve replacement (ACE) or transcatheter aortic valve implantation (TAVI) every year. In the majority of cases, the primary indication is calcifying aortic valve stenosis, which affects 3-5% of the population older than 75 years [1].
Tachyarrhythmias, particularly atrial fibrillation (AF) and atrioventricular (AV) conduction disorders, especially left bundle branch block (LSB) and higher-grade AV block, are among the most common rhythm problems before, during, and after surgical AKE or TAVI. These arrhythmias increase both morbidity and mortality, lead to prolonged hospital stays, and increase costs.
The clinical impact of these arrhythmias in patients with aortic valve vitium is varied, with symptoms ranging from complete absence of symptoms or discomfort to occasional palpitations, fatigue, dizziness, dyspnea, or chest pain to severe clinically manifest heart failure, syncope, cardiogenic shock, and death.
Atrial fibrillation – Seek and you shall find!
Atrial fibrillation is the most common cardiac arrhythmia with steeply increasing prevalence with age: in those over 80 years of age, the prevalence is already over 10% [1]. The prevalence is additionally increased in renal failure, COPD, heart failure, and in patients undergoing valve surgery (Fig. 1) [2]. In patients with severe aortic valve stenosis, this prevalence increases again significantly and, according to the literature, is 8-13% before surgical AKE and 16-51% before TAVI [3].
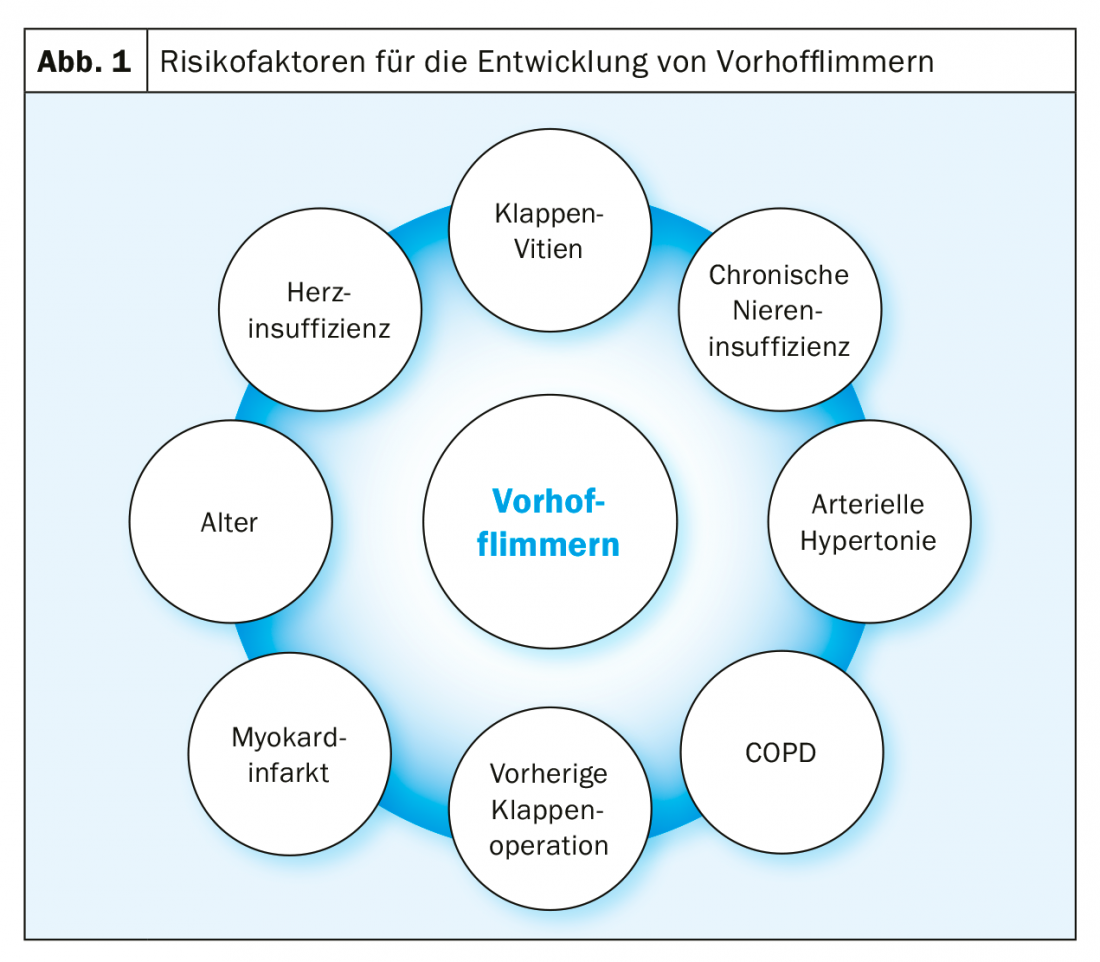
This very high prevalence of VCF in severe aortic valve stenosis is due, on the one hand, to the co-incidence of risk factors for both diseases: in general, patients with severe aortic valve stenosis are mostly very elderly. On the other hand, the vitium itself also causes hemodynamic changes leading to left atrial pressure and consecutive left atrial fibrosis with also electrical changes in the atria. This substrate transformation overall favors the occurrence of VHF (Fig. 2).
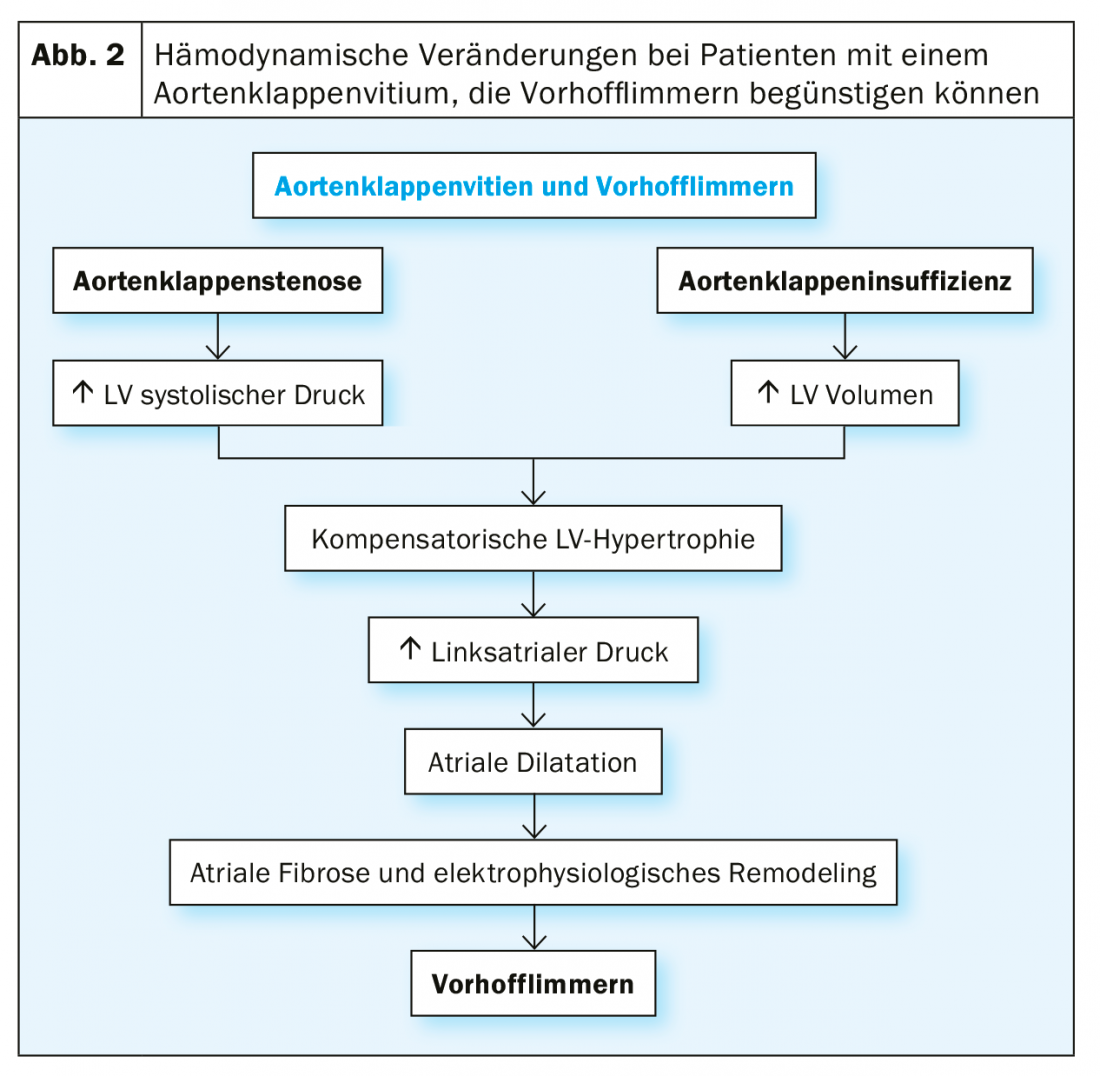
In particular, surgical AKE generates a hyperadrenergic state periinterventionally and the surgical trauma induces a cardiac inflammatory response, which is considered to be partly responsible for the development of periinterventional VCF.
Even after surgical AKE or TAVI, VHF is the most common newly diagnosed arrhythmia. The incidence here is strongly dependent on the screening method used: the longer rhythm monitoring is performed, the more frequently VCF is found.
Basically, high incidence rates of VCF are described after surgical AKE and TAVI (31-64%, and 4-32%, respectively) [4]. A recently published study by Kalra et al, which analyzed data from 171,480 patients after surgical AKE and TAVI, found an incidence rate of VCF by approximately 50% in both groups [2]. In the important NOTION trial, 52 patients were continuously monitored by an event recorder after surgical AKE or TAVI [4]. The incidence of VCF was 100% after surgical AKE and 82% after TAVI. After surgical AKE, the new diagnosis of AF occurred within the first 61 days in all patients and after TAVI within the first 41 days. Arrhythmia burden in the first 2 weeks after surgical AKE and TAVI was significantly longer at 2.8% in patients after surgical AKE compared to 0.04% after TAVI (p=0.01). However, arrhythmia burden decreased significantly after 3 months in patients after surgical AKE [4].
It is likely that VHF was preexisting but asymptomatic in many of these patients. Underdiagnosis of asymptomatic AF is a general problem and does not only affect patients with severe aortic valve stenosis.
A new diagnosis of VCF after surgical ACE or TAVI results in a prolongation of hospitalization compared to patients without VCF (9 vs. 6 days for both groups; p<0.001) [2]. In these patients, a significantly increased mortality during hospitalization after surgical ACE and TAVI can also be observed compared with patients without newly diagnosed VCF. The 1-year mortality of patients with incisional VCF after TAVI is also significantly increased compared with patients without newly diagnosed VCF (31% vs. 14%; p<0.01) [5].
Oral anticoagulation for thromboembolism prophylaxis should be used in all patients with VHF and increased risk of thromboembolism.
AV conduction disorders and pacemaker requirements
The most important risk factors for AV conduction disorders are age, heart failure, coronary artery disease, arterial hypertension, and diabetes mellitus [6]. It is therefore not surprising that AV conduction defects are very common in the typically rather elderly patients with severe aortic valve stenosis: in 10-20% of patients receiving surgical AKE or TAVI, a pacemaker has been implanted beforehand [7]. Additional risk factors for AV conduction dysfunction after TAVI include, in particular, complete right bundle branch block and 1st degree AV block (Fig. 3).
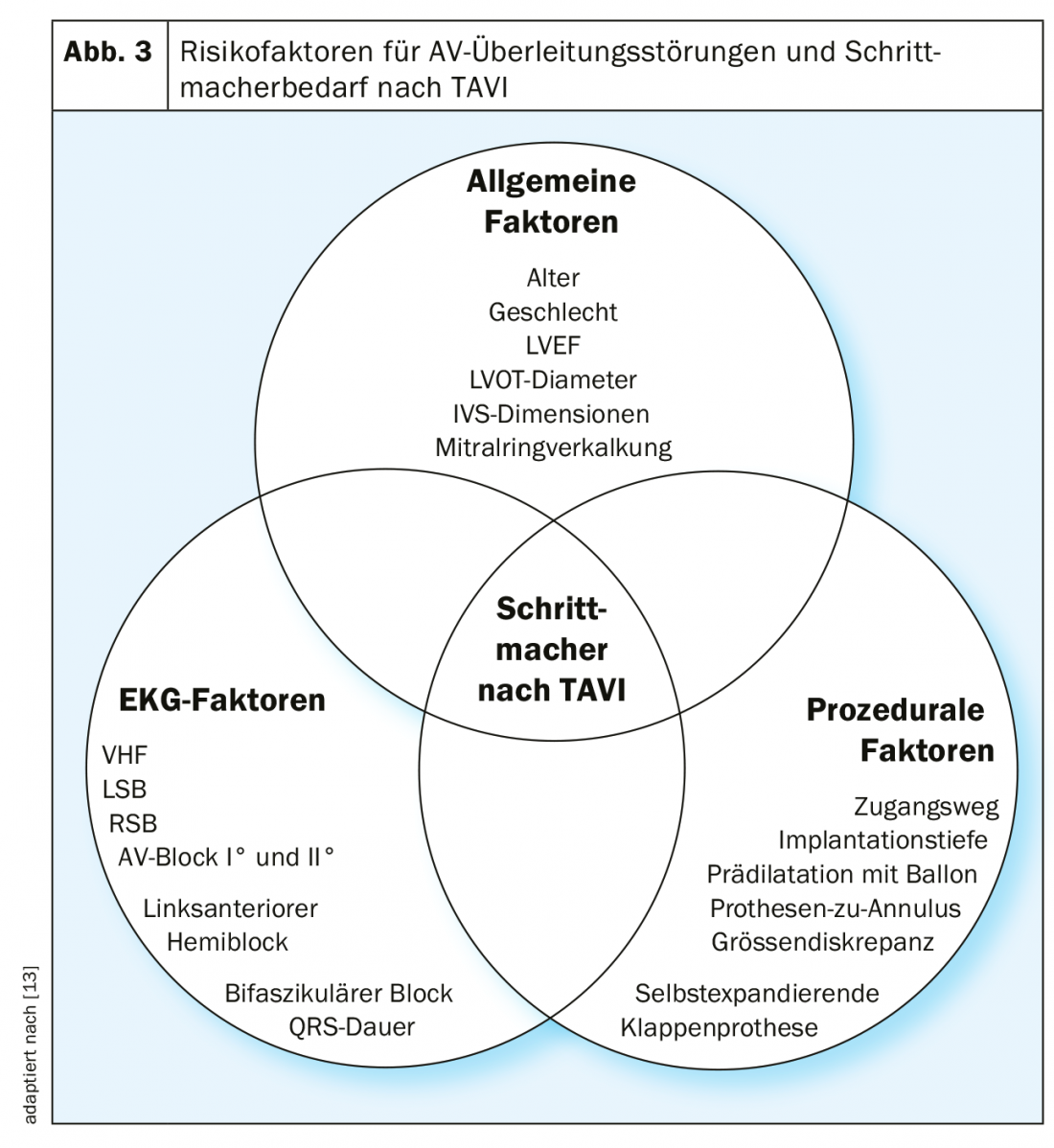
The development of AV conduction defects during and after surgical AKE or TAVI is also due to the close anatomic relationship of the aortic valve with the conduction system. Thus, the left fascicle runs relatively superficially just below the aortic valve in the left ventricular outflow tract. Mechanical compression of this region by a deeply implanted aortic valve during TAVI, or injury to this region during surgical AKE, leads to left bundle branch block or even complete AV block, the latter especially in the presence of preexisting right bundle branch block.
If a bicuspid aortic valve or a severely calcified aortic valve stenosis is present, a higher prevalence of AV conduction abnormalities and pacing requirements is also observed because of the need for extensive debridement.
The incidence of AV conduction defects after TAVI is also dependent on the implanted valve model. This is generally higher for self-expandable valve prostheses compared to balloon-expandable models. The size of the aortic valve annulus in relation to the diameter of the prosthetic valve also plays a role in addition to the implantation depth.
Atrioventricular conduction abnormalities most commonly occur during or shortly after valve implantation, but may occur several days after TAVI [8]. The latter makes the management of these patients difficult and leads to a much more liberal indication for pacemaker implantation after TAVI compared with surgical AKE. In contrast, after surgical AKE, up to one week is waited in case of complete AV block and a pacemaker is implanted only if the AV block fails to recover.
The incidence of left bundle branch block after surgical AKE is 3-4% and the incidence of persistent complete AV block with pacing requirements is 3-12% [8,9]. In contrast, the incidence of left bundle branch block after TAVI is 18-65% for self-expandable and 4-30% for balloon-expandable prosthetic valves [10]. A pacemaker is reimplanted in 25-28% of patients after TAVI with a self-expandable prosthetic valve and in 5-7% of patients who received a balloon-expandable prosthetic valve [10].
It is intuitive to assume that complete left bundle branch block is prognostically unfavorable because of the associated ventricular dyssynchrony, especially in the presence of concomitant impaired left ventricular function. Similarly, iatrogenic new left bundle branch block carries a risk of progression of AV conduction disturbance to complete AV block.
In a meta-analysis, Regueiro et al. describe. In patients with new left bundle branch block after TAVI, a higher rate of pacemaker implantation (RR 2.18; 95% CI, 1.28-3.70; p<0.01), higher cardiac mortality (RR 1.39, 95% CI, 1.04-1.86, p=0.03), and a negative effect on left ventricular pump function within the first year after TAVI compared to patients without new left bundle branch block [11]. However, data are conflicting in this regard: another meta-analysis, for example, failed to demonstrate increased mortality in patients with new left bundle branch block after TAVI [12].
Providing the right patients with a pacemaker at the right time after TAVI remains a difficult clinical challenge.
Literature:
- Go AS, Hylek EM, Phillips KA, et al: Prevalence of Diagnosed Atrial Fibrillation in Adults. Jama 2001; 285: 2370.
- Kalra R, Patel N, Doshi R, et al: Evaluation of the Incidence of New-Onset Atrial Fibrillation After Aortic Valve Replacement. JAMA Intern Med 2019; 35294: 1-9.
- Tarantini G, Mojoli M, Urena M, Vahanian A: Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: epidemiology, timing, predictors, and outcome. Eur Heart J 2017; 38: 285-293.
- Jørgensen TH, Thyregod HGH, Tarp JB, et al: Temporal changes of new-onset atrial fibrillation in patients randomized to surgical or transcatheter aortic valve replacement. Int J Cardiol Elsevier B.V.; 2017; 234: 16-21.
- Stortecky S, Buellesfeld L, Wenaweser P, et al: Atrial fibrillation and aortic stenosis. Circ Cardiovasc Interv 2013; 6: 77-84.
- Kerola T, Eranti A, Aro AL, et al: Risk Factors Associated With Atrioventricular Block. JAMA Netw open 2019; 2: e194176.
- Franzone A, Windecker S: The Conundrum of Permanent Pacemaker Implantation after Transcatheter Aortic Valve Implantation. Circ Cardiovasc Interv 2017; 10: 1-4.
- Roten L, Stortecky S, Scarcia F, et al: Atrioventricular conduction after transcatheter aortic valve implantation and surgical aortic valve replacement. J Cardiovasc Electrophysiol 2012; 23: 1115-1122.
- Khounlaboud M, Flécher E, Fournet M, et al: Predictors and prognostic impact of new left bundle branch block after surgical aortic valve replacement. Arch Cardiovasc Dis Elsevier Masson SAS; 2017; 110: 667-675.
- Auffret V, Puri R, Urena M, et al: Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation 2017; 136: 1049-1069.
- Regueiro A, Altisent OAJ, Trigo M Del, et al: Impact of new-onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement. Circ Cardiovasc Interv 2016; 9: 1-10.
- Ando T, Takagi H: The Prognostic Impact of New-Onset Persistent Left Bundle Branch Block Following Transcatheter Aortic Valve Implantation: A Meta-analysis. Clin Cardiol 2016; 39: 544-550.
- Siontis GCM, Jüni P, Pilgrim T, et al: Predictors of Permanent Pacemaker Implantation in Patients With Severe Aortic Stenosis Undergoing TAVR. J Am Coll Cardiol Elsevier Inc; 2014; 64: 129-140.
CARDIOVASC 2019; 18(5): 10-12