The years 2020 and 2021 have brought some novelties in metastatic prostate cancer. Thus, with 177Lutetium-PSMA-617and the PARP inhibitor Olaparib, two new active substances are available. The latter is already approved in Switzerland, and therapy using 177Lutetium-PSMA-617is accessible under a compassionate use program. Thus, for patients with metastatic castration-refractory prostate cancer, new options exist after failure of first-line therapy, which also increase the value of genetic testing in this entity.
The years 2020 and 2021 have brought some novelties in metastatic prostate cancer (mPCa). At this year’s American Society of Oncology (ASCO) Annual Meeting, a study in metastatic castration-refractory prostate cancer (mCRPC) was presented in the plenary session (VISION Trial to 177Lutetium-PSMA). Similarly, a study (PROfound) of the PARP inhibitor olaparib was presented at the European Society for Medical Oncology(ESMO) 2020 Congress and has already led to approval. This represents the high level of scientific input in this tumor entity. Meanwhile, the new findings and subsequent treatment options enable a large number of patients to survive for a long time despite metastatic disease.
Unfortunately, as reported in various studies from the hormone-sensitive setting, not all patients respond for up to two years to combined systems therapy with androgen deprivation (ADT) plus androgen receptor (AR)-targeted agent [1– 4] or ADT plus docetaxel [5]. There are also rapid courses with progression as early as six months. For these patients, the development of potent therapies is also needed at the mCRPC stage. Here, two new therapies were added to the armamentarium in 2020 and 2021. One of these, that using olaparib, is already approved in the setting of mCRPC, in molecularly selected BRCA1/2 mutated patients after failure of a Novel Hormonal Agent (NHA) or in addition to taxane-containing chemotherapy (CHT) (not mandatory) [6,7].
News metastatic castration-resistant prostate cancer (mCRPC).
1. targeting PSMA – PSMA PET CT and lutetium-PSMA(177Lu-PSMA-617).
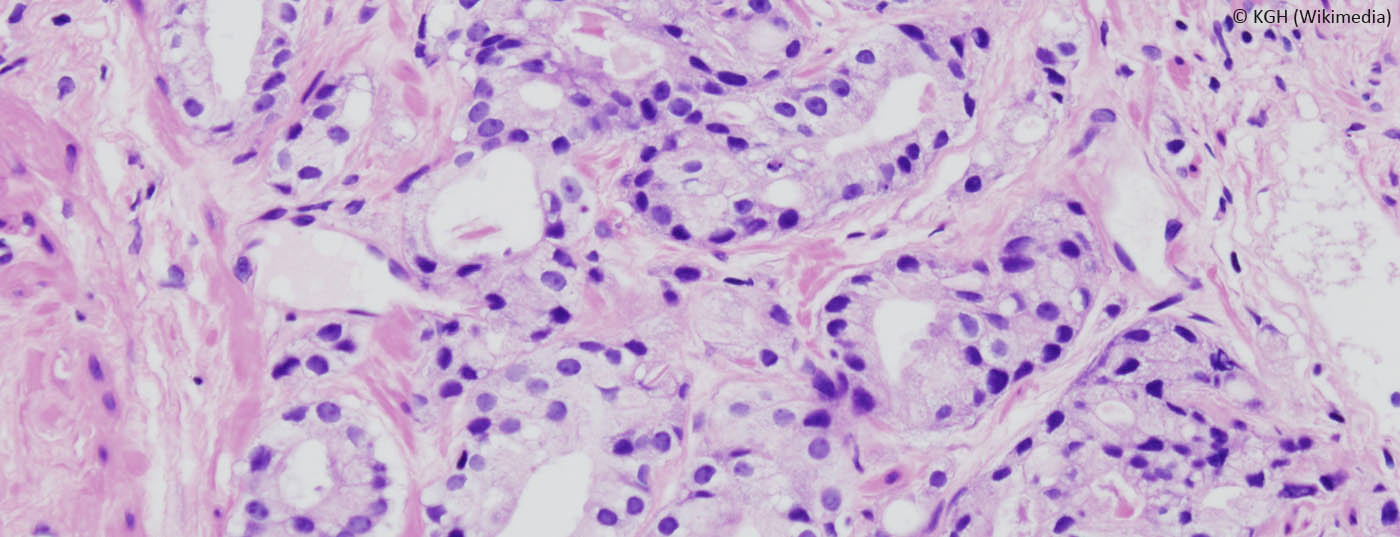
Prostate-specific membrane antigen (PSMA) is a membrane-bound glycoprotein that is highly expressed in a high proportion of patients (up to 75%) in mCRPC [8]. Furthermore, PSMA expression has been shown to increase during the progression from benign prostatic hyperplasia (BPH) to invasive prostate cancer and correlates accordingly with Gleason score and disease aggressiveness [9]. PSMA expression is highly dependent on the AR pathway and can be upregulated by ADT and modern AR-targeting agents such as enzalutamide [10,11]. Appropriate clinical trial protocols are currently investigating the combination of therapy directed against 177PSMAand oral AR-targeting agents (ENZA-p, NCT04419402).
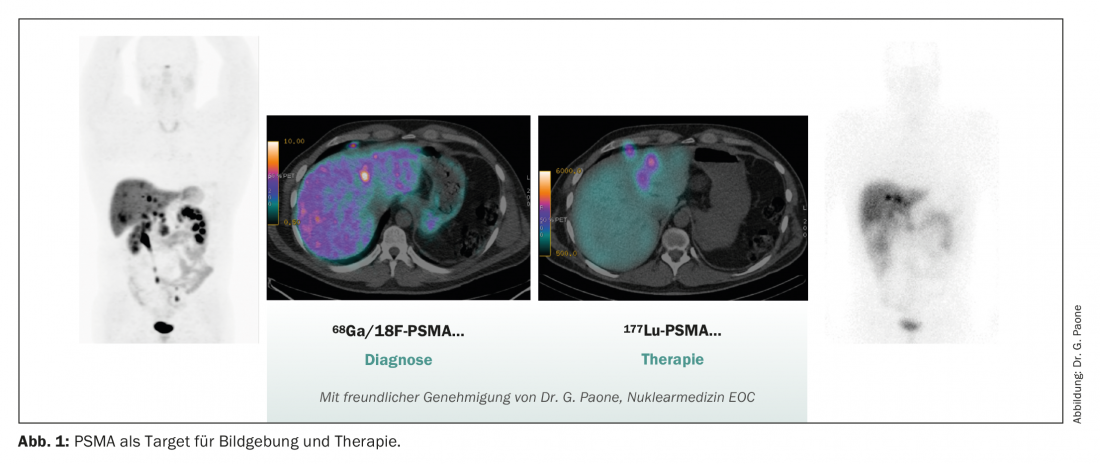
PSMA PET-CT imaging with 66Ga-PSMAis now widely used in Switzerland and has therefore been implemented and approved for staging of high-risk localized prostate carcinomas or biochemical recurrence after definitive local treatment and for assessment of suitability for treatment with 177Lu-PSMA. The application in high-risk localized prostate carcinoma is new. Here, a phase III trial demonstrated that PSMA PET-CT had significantly higher sensitivity and specificity for lymph node positivity and the detection of distant metastases compared with conventional staging using CT or bone scintigraphy. This had a significant impact on therapy management in the study.
In about a quarter of cases, there was a switch from potentially curative to palliative therapy after distant metastases were found on PSMA PET-CT [12]. Whether this additional knowledge thanks to PSMA PET-CT, often with upstaging, has an impact on survival or is cost-effective is currently under investigation.
PSMA is playing an increasingly important role not only in diagnostics, but also in therapy. With the positive phase III trial data of 177LutetiumPSMA-617 in the VISION trial, another treatment option will soon be available in pretreated mCRPC patients [13]. The study enrolled 831 mCRPC patients with PSMA PET-positive lesions who were pretreated with at least one new hormonal agent (NHA) and one taxane-based chemotherapy line. After randomization, patients received up to six cycles of 177Lu-PSMA-617as a six-week infusion with protocol-permitted standard of care (SOC) compared with SOC alone (mainly consisting of glucocorticosteroids or a second NHA, as cabazitaxel, radium-223, or investigational drugs were not allowed). The study met its primary endpoint of overall survival (OS) with a median OS of 15.3 versus 11.3 months (HR 0.62, 95% CI: 0.52 to 0.74; p<0.001). Adverse reactions > grade 3 were observed in 52.7% of patients. Thrombocytopenia (8% grade 3) and anemia (13% grade 3) were most common. In general, adverse drug reactions were well controlled, so quality of life was usually not affected.
In Switzerland, there are a few selected centers, including our nuclear medicine department in Bellinzona, which have been able to offer patients treatment using 177LutetiumPSMA-617 for the past year as part of a compassionate use program. As a result, we have already been able to gain good expertise in clinical practice. Regulatory approvals by Swissmedic in Switzerland and by the EMA in the EU are still pending. Therefore, personalized treatment, which is reserved for patients with highly PSMA-expressing metastases, is currently considered the new standard of care in pretreated mCRPC patients.
The intensity of PSMA expression is likely to be of prognostic value for response to 177LutetiumPSMA-617 therapy, according to initial study results. Thus, low average PSMA expression proved to be unfavorable for response, but a precise definition of the SUV cut-off is still lacking [14].in the phase II TheraP trial, participants were also required to undergo FDG PET as part of the screening process to detect discordant FDG-storing lesions and PSMA PET-negative lesions that precluded study participation [15]. In the Vision Trial, approximately 20% of patients were excluded from therapy due to inadequately storing lesions on PET PSMA-CT. In clinical practice, patients should be well selected, because those with very advanced disease (superscan of bone scintigraphy or extensive liver metastasis) and low bone marrow reserve do not benefit from the nevertheless very complex and cost-intensive treatment with 177LutetiumPSMA-617.
2. poly-ADP-ribose polymerase (PARP) inhibitors in selected mCRPC patients with somatic or genomic alterations in repair genes (HRD) – focus on breast cancer genes (BRCA) 1 and 2.
PARP inhibitors have become an integral part of the treatment landscape for some solid tumors, such as ovarian or breast carcinoma. Due to the widespread use, experience with the side effect profile and effectiveness can already be gathered for quite some time. Alterations in DNA repair genes such as BRCA1 and BRCA2 are also present in a significant number of prostate cancer patients and serve as a target.
It has recently been reported in the literature that somatic BRACA1 mutations are present in mCRPC in circa 9%, and somatic BRCA2 mutations are present in about 2% [16]. Germline mutations are more common in patients with advanced PCa (11.8%) than in the general PCa population [17]. Therefore, current guidelines recommend an early search for alterations or mutations of DNA repair genes (at least BRCA1/2) in tumor tissue – and thus somatic testing. This should be done at the latest at the beginning of the castration-refractory stage, since from this point on therapy with the PARP inhibitor olaparib is now also approved by Swissmedic. EMA and FDA approvals are also available if a BRCA1 or 2 mutation can be detected.

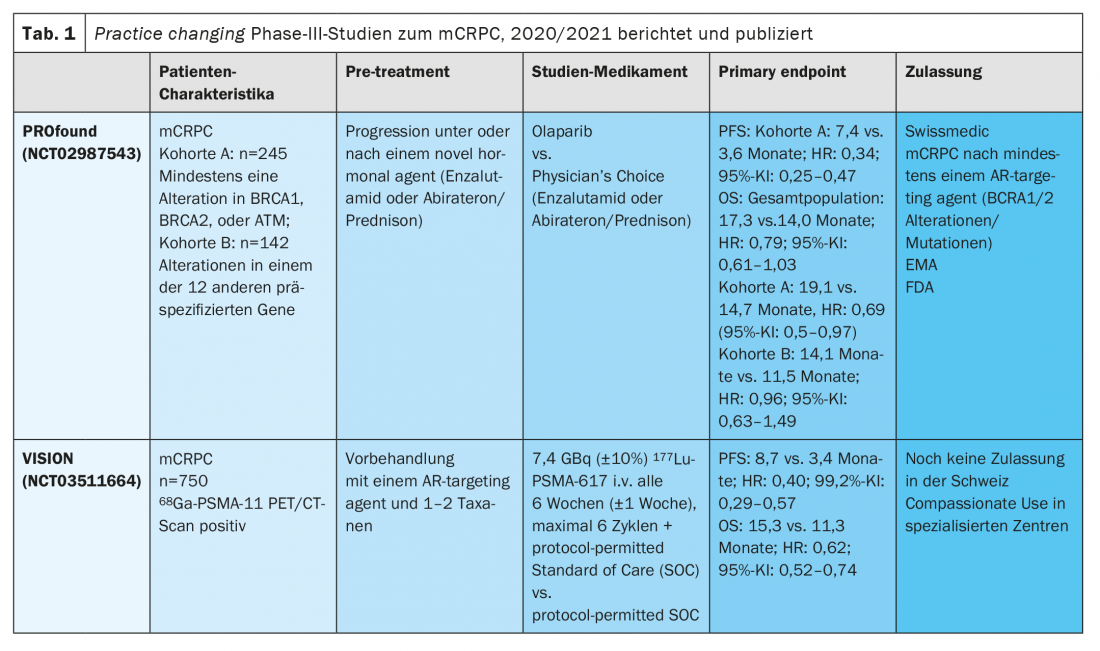
Olaparib was approved in Switzerland earlier this year based on the results of the randomized Phase III PROfound study. In this randomized-controlled trial, patients with mCRPC received therapy with either olaparib or Investigator’s Choice (enzalutamide or abiraterone/prednisone).
The study met its primary endpoint of progression-free survival (PFS) and additionally also met the key secondary endpoint of OS in patients with defects in 15 predefined HRR genes [6,7]. The PROfound trial was designed with two cohorts in the olaparib arm, with cohort A including patients with the most common defects in DNA repair genes such as BRCA1 and 2 and ATM, and cohort B including sufferers with DNA repair defects in other 12 previously specified genes. After radiographic progression in the control arm, crossover to olaparib was allowed. Although the study was positive for all endpoints in cohort A (median OS 19.1 vs. 14.7 months; HR: 0.69 (95% CI: 0.5-0.97), exploratory genetic analysis showed significant benefit only in patients with mutations or alterations of BRCA1/2 (HR 0.63, 95% CI: 0.42-0.95). The hazard ratio (HR) for olaparib in ATM mutations was 0.93 (95% CI: 0.53-1.75). As a result, the benefit in the presence of an ATM mutation is small. The bottom line is that these results led to regulatory approval of olaparib only for mCRPC patients with BRCA1/2 mutations and at least one prior treatment with an AR-targeting agent. The toxicity profile was consistent with previous reports.
Take-Home Messages
- With 177Lutetium-PSMA-617and the PARP inhibitor Olaparib, two new agents are available for the treatment of metastatic castration-refractory prostate cancer.
- There is currently no approval for 177Lutetium PSMA therapy
- in Switzerland, but it is accessible as part of a compassionate use program at specialized centers. Treatment of metastatic castration-refractory prostate cancer with olaparib has been available since 2020 following approval.
- 177Lutetium-PSMA therapyis used after failure of an AR-targeting agent and a taxane (docetaxel). The ideal therapeutic sequence with cabazitaxel (an established standard after docetaxel failure) is currently unclear and needs to be assessed on an individual basis.
- The PARP inhibitor olaparib is approved for the treatment of metastatic castration-refractory prostate cancer with BRCA1/2 mutation after pretreatment with at least one novel hormonal agent. Testing for somatic and/or germline mutations of DNA repair genes is indicated in mCRPC no later than the time when the patient would be eligible for olaparib treatment.
Literature:
- Fizazi K, et al: Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. New England Journal of Medicine. 2017; 377: 352-360.
- Chi KN, et al: TITAN: A randomized, double-blind, placebo-controlled, phase 3 trial of apalutamide (ARN-509) plus androgen deprivation therapy (ADT) in metastatic hormone-sensitive prostate cancer (mHSPC). Annals of Oncology. 2016; 27: vi265.
- Davis ID, et al: Enzalutamide with standard first-line therapy in metastatic prostate cancer. New England Journal of Medicine. 2019; 381: 121-131.
- James ND, et al: Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. New England Journal of Medicine. 2017; 377: 338-351.
- Sweeney CJ, et al: Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. New England Journal of Medicine. 2015; 373: 737-746.
- de Bono J, et al: Olaparib for Metastatic Castration-Resistant Prostate Cancer. New England Journal of Medicine. 2020; 382: 2091-2102.
- Hussain M, et al: Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. New England Journal of Medicine. 2020; 383: 2345-2357.
- Vlachostergios PJ, et al: Prostate-Specific Membrane Antigen Uptake and Survival in Metastatic Castration-Resistant Prostate Cancer. Frontiers in Oncology. 2021; 11.
- Sweat SD, et al: Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998; 52: 637-640.
- Wright GL, et al: Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996; 48: 326-34.
- Rosar F, et al: New insights in the paradigm of upregulation of tumoral PSMA expression by androgen receptor blockade: enzalutamide induces PSMA upregulation in castration-resistant prostate cancer even in patients having previously progressed on enzalutamide. Eur J Nucl Med Mol Imaging. 2020; 47: 687-694.
- Hofman MS, et al: Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. The Lancet. 2020; 395: 1208-1216.
- Sartor O, et al: Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. New England Journal of Medicine. 2021. doi: 10.1056/NEJMoa2107322. Epub ahead of print.
- Seifert R, et al: Analysis of PSMA expression and outcome in patients with advanced Prostate Cancer receiving 177Lu-PSMA-617 Radioligand Therapy. Theranostics. 2020; 10: 7812-7820.
- Hofman MS, et al: 177Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. The Lancet. 2021; 397: 797-804.
- Mahal BA, et al: Racial Differences in Genomic Profiling of Prostate Cancer. New England Journal of Medicine. 2020; 383: 1083-1085.
- Pritchard CC, et al: Inherited DNA repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016; 375: 443-453.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(4): 6-9.