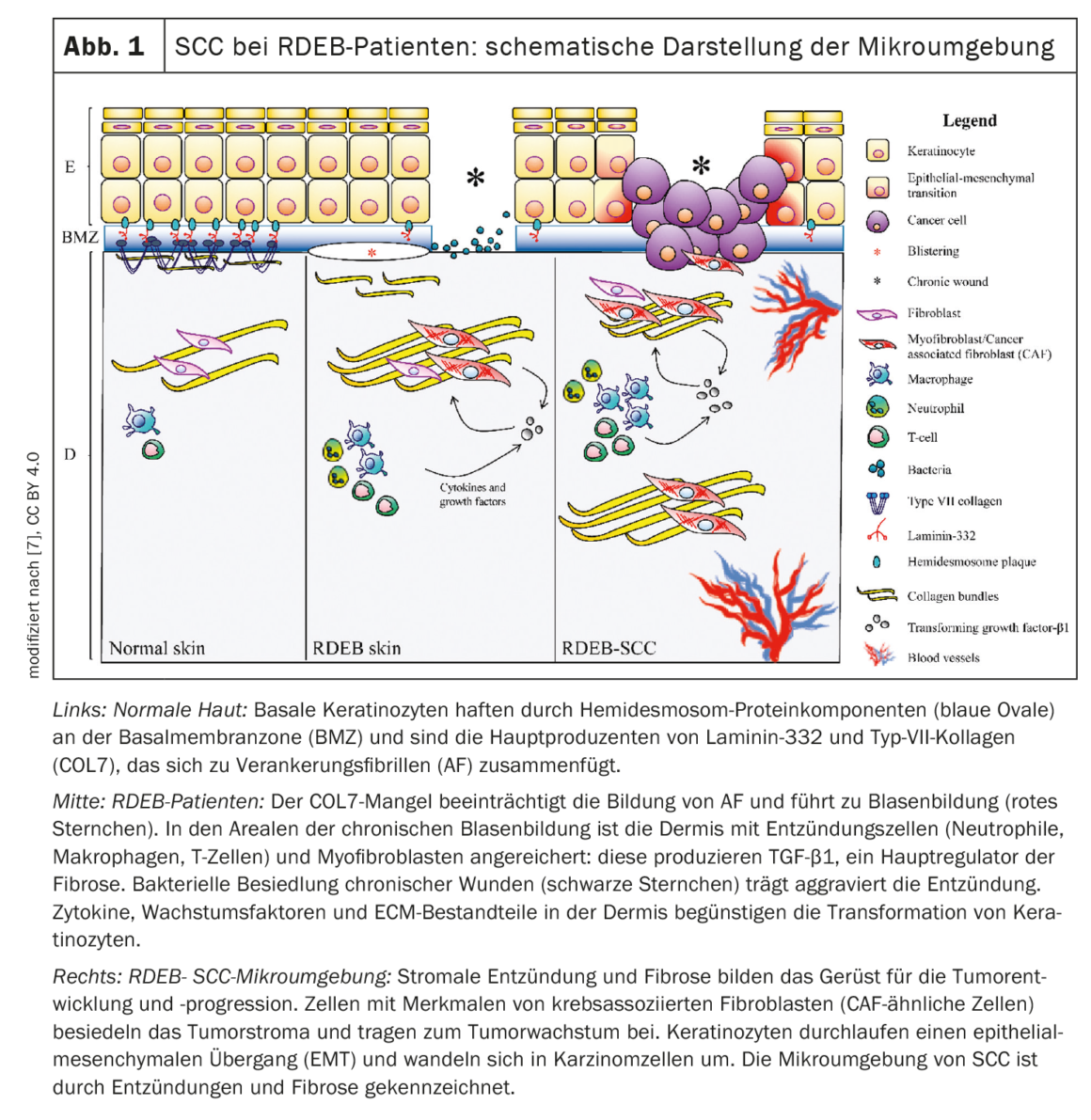
Bullous autoimmune dermatoses result from an autoantibody-driven immune response against structural proteins of the skin. Chronic wounds in the setting of recessively inherited dystrophic epidermolysis bullosa (RDEB) are highly susceptible to malignant transformation into life-threatening squamous cell carcinomas of the skin. Researchers are combining molecular and cellular profiling techniques with functional assays to identify markers that may one day be used for diagnosis and therapy.
Epidermolysis bullosa hereditaria (EB) subsumes a group of diseases characterized by pathological fragility of the skin and mucous membranes, accompanied by blistering and erosions. To date, over a thousand mutations in 20 different genes are known to be associated with EB [1]. These are predominantly genes encoding structural proteins in the epidermis or at the dermoepidermal junction. Depending on the depth of the morphological bubble formation level, the following 4 EB types are mainly distinguished [2]:
- EB simplex (EBS suprabasal and basal)
- junctional EB (JEB)
- dystrophic EB (DEB)
- Kindler syndrome
A mutation in COL7A1 is considered to be the cause of DEB. The latter encodes an important component of anchoring fibrils, collagen VII [1]. According to its mode of inheritance, DEB is divided into dominant (DDEB) and recessive DEB (RDEB). RDEB is characterized by epidermal fragility, trauma-induced blistering, and long-term wounds that are difficult to heal. In generalized, severe forms of RDEB, squamous cell carcinoma (SCC) is disproportionately common due to chronic recurrent inflammatory tissue trauma and permanent reactive regeneration/hyperproliferation.
RDEB is associated with early manifestation of SCC.
The risk of shortened life expectancy due to SCC-related metastases is more than 87% in patients with RDEB, explained Prof. Dr. Iris Gratz, Biosciences and Medical Biology, Paris Lodron University, Salzburg (A) [2]. The risk increases in an age-correlated manner and depending on the form of progression, but is already much higher in those under 40 years of age than in the general population (box). The fibrosis that develops rapidly in RDEB skin contributes to both chronic wounds and squamous cell carcinoma – the leading cause of death in this patient group [3]. As in other fibrotic diseases, certain molecular signaling pathways are disrupted in RDEB [3]. Researchers led by Prof. Gratz are investigating the role of cutaneous T cells using 3D organotypic skin cultures, mouse models of T cell-mediated skin inflammation, and xenografting mouse models. The initial goal is to uncover unknown and underappreciated factors that contribute to wound chronicity, malignant transformation, and tumor progression in order to hopefully use this knowledge later for diagnosis and therapy. Recently, researchers have uncovered the recirculation behavior of human skin-resident memory T cells [4], functionally and transcriptionally analyzed circulating human CD4+ T cells in vivo [5], and developed a novel skin-humanized mouse model to study T cell migration and activation of human skin TRM in vivo [6].
| RDEB-associated spinaliomas: age-correlated increase in risk. Squamous cell carcinoma (SCC) associated with RDEB is more aggressive and characterized by higher morbidity and mortality than SCC in the general population. SCC represent the leading cause of death in patients with severe generalized RDEB. The cumulative risk for these patients to develop at least one spinalioma is already 67.8% at age 35. At age 55, the risk increases to 90.1% (National EB Registry, USA). Typically, SCC develop in areas of chronic wounds and scars, especially in the extremities. |
Cutaneous and blood circulating TRM cells in focus.
In summary, at this time, RDEB is an inflammatory disease entity that is associated with a massively increased risk of squamous cell carcinoma (SCC) [1]. Empirical studies have shown that cutaneous T cells produce interleukin (IL)17 and IL22 at elevated levels. Furthermore, cutaneous tissue-resident memory T cells ( TRM cells), were found to be able to leave the skin and circulate in the bloodstream [1]. And SCC was found to be associated with a significantly increased fraction of IL17A-producing circulating TRM, whereas this was not the case for other cytokines such as IL13, GM-CSF, or IFN-gamma. Furthermore, it was observed that tumor-associated fibroblasts have a Th17-regulated transcriptome. Based on these findings, it is currently believed that T cell-derived IL17 and IL22 can drive tumor growth both directly and indirectly via fibroblasts (e.g., by triggering pro-inflammatory processes) [1]. To determine whether these pathways can be blocked, several studies are currently underway using 3-D skin models, SCC cultures, and SCC xenografting models. Among other findings, single cell analysis of RDEB tissue revealed that increased expression of IL17A is present in SCC-affected RDEB tissue compared to tumor-free RDEB skin samples [1].
Congress: ADF Annual Meeting
Literature:
- Schweiger-Briel A, et al: Epidermolysis Bullosa – Overview and Swiss network. Paediatrica 2018; 29(3): 36-34.
- “Skin inflammation and cancer – Can we learn from rare genetic diseases?”, Dr. Iris Gratz, ADF Annual Meeting, Innsbruck, Feb. 24, 2023.
- Tartaglia G, et al: Impaired Wound Healing, Fibrosis, and Cancer: The Paradigm of Recessive Dystrophic Epidermolysis Bullosa. Int J Mol Sci 2021; 22(10): 5104.
- Klicznik MM, et al: Human CD4+CD103+ cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci Immunol 2019; 4(37).
- Höllbacher B, et al: Transcriptomic Profiling of Human Effector and Regulatory T Cell Subsets Identifies Predictive Population Signatures. Immunohorizons 2020; 4(10): 585-596.
- Klicznik MM, et al: A novel humanized mouse model to study the function of human cutaneous memory T cells in vivo in human skin. Sci Rep 2020; 10(1): 11164.
- Condorelli AG, et al: Epidermolysis Bullosa-Associated Squamous Cell Carcinoma: From Pathogenesis to Therapeutic Perspectives. Int J Mol Sci 2019; 20(22): 5707. www.mdpi.com/1422-0067/20/22/5707,(last accessed May 23, 2023) .
- Montaudié H, et al: Inherited epidermolysis bullosa and squamous cell carcinoma: A systematic review of 117 cases. Orphanet. J Rare Dis 2016; 11: 11.
- Fine JD, et al: Epidermolysis bullosa and the risk of life-threatening cancers: the National EB Registry experience, 1986-2006. JAAD 2009; 60: 203-211.