Typical symptoms of chronic rhinosinusitis with nasal polyps (CRSwNP) are obstructed nasal breathing, secretions, facial pain and olfactory disturbances. If adequate therapy is not provided, there is a risk of dangerous complications. For a long time, treatment options were limited to topical or systemic steroids and endoscopic sinus surgery. However, biologics have now also been approved for several years.
Treatment of CRSwNP with biologics is a promising therapeutic option for severe cases that do not respond adequately to conventional treatment [1]. CRSwNP is a multifactorial disease of the nasal and paranasal mucosa, often with underlying type 2 inflammation. Rhinosinusitis is considered chronic if there is persistent symptomatic inflammation of the mucous membrane of the nose and paranasal sinuses for more than twelve weeks, characterized by nasal obstruction or swelling or rhinorrhea (at least one of these) with facial pain or a feeling of pressure and/or loss of sense of smell (in adults) or coughing (in children) [2]. For the phenotype with nasal polyp formation (CRSwNP), endoscopic and/or radiological evidence of polypous-hyperplastic tissue in the nasal cavity and/or paranasal sinuses is mandatory [2]. “We know that some of the patients have type 2 inflammation,” reported Prof. Dr. med. Michael Soyka, Head Physician, Department of Ear, Nose, Throat and Facial Surgery, University Hospital Zurich. “As we now have a better understanding of the pathophysiology, the use of modern biologics is also an option,” said the speaker [3].
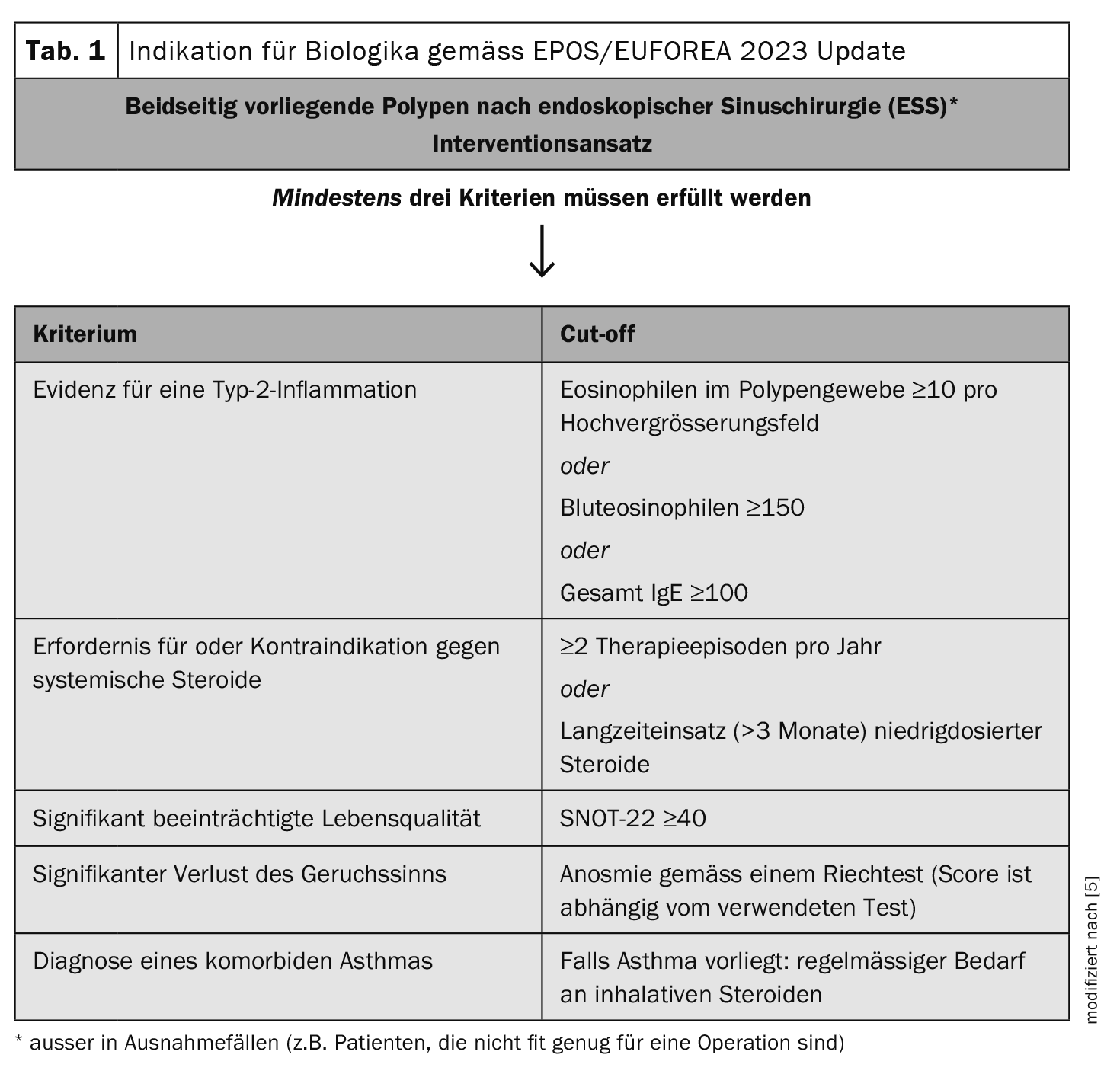
Which criteria are decisive?
Indicators for the endotype of an underlying type 2 inflammation are blood eosinophils (>0.15/0.25/0.30 G/l) or a total IgE >100/150kU/l or a histology with >10 eosinophils per high magnification field. Further evidence is provided by the response to oral steroids and the presence of eosinophilic comorbidities such as adult-onset asthma [3]. For CRSwNP patients with type 2 inflammation, a monoclonal antibody can be used in addition to the classic treatment options (intranasal topical steroids, oral steroids). In Switzerland, three biologics (omalizumab, dupilumab, mepolizumab) are currently approved for this indication.
An EPOS/EUFOREA position paper on the use of biologics published in 2023 proposed the following changes to the recommendations issued by EPOS in 2020 [4,5]. In line with the current specialist literature, the cut-off value for eosinophils in the blood was reduced from ≥250 cells/ml to ≥150 cells/ml. There was a discussion as to whether other inflammatory diseases mediated by type 2 inflammation should be included as criteria for biologic therapy in addition to asthma. Although biologics have been shown to be effective in many forms of type 2 disease, the lack of association between CRSwNP and allergic rhinitis [4]. and the ambiguous data situation regarding CRSwNP and atopic dermatitis or eosinophilic esophagitis [4,6–9] led to the decision not to include other diseases mediated by type 2 inflammation as a criterion for the indication.
In the Swiss healthcare system, several conditions must be met in order to prescribe a biologic for CRSwNP [3,10]. Specifically, the following five criteria are mandatory:
- Nasal polyps on both sides
- Surgery/corticosteroids: recurrence within two years, at least one surgery and failure (or contraindications) of topical and systemic corticosteroids
- Endoscopic nasal polyp score (NPS): at least 5 out of 8; at least two per nasal cavity
- According to SNOT-22 (Sino-Nasal Outcome Test-22) significantly impaired quality of life (SNOT-22 ≥50)
- Symptoms for at least 12 weeks: nasal congestion (NC), moderate or high severity (score of 2 or 3):
In addition, at least one of the following criteria must apply:
- since at least 12 weeks of partial or complete loss of smell (hyposmia/anosmia) according to the Sniffin’ Sticks Test with 16 items ≤10 points or UPSIT score ≤25
- since at least 12 weeks of rhinorrhea (anterior or posterior)
The efficacy and safety of monoclonal antibodies against IL-4/IL-13 (dupilumab), IgE (omalizumab) and IL-5 (mepolizumab) in CRSwNP have been demonstrated in the respective phase III studies [11–13]. The primary endpoints of these studies were reduction in nasal polyp score (NPS) and nasal congestion/obstruction score. The verum arms proved to be clearly superior in the placebo comparison [14]. Significant improvements were also achieved with all three biologics in other measures such as sense of smell and disease-specific quality of life (SNOT-22). It is worth noting that a significant proportion of patients with comorbid asthma (48-71%) and previous sinus surgery (58-100%) were included in these Phase III trials.
Four network meta-analyses (NMA) published in 2021-2022 performed indirect comparisons between the different monoclonal antibodies [15, 16-19].
- Agache et al: Efficacy and safety of treatment with biologicals for severe chronic rhinosinusitis with nasal polyps: a systematic review for the EAACI guidelines. Allergy 2021; 76(8): 2337-2353.
- Oykhman et al: Comparative efficacy and safety of monoclonal antibodies and aspirin desensitization for chronic rhinosinusitis with nasal polyposis: a systematic review and network meta-analysis. J Allergy Clin Immunol 2021; S0091-6749(21):01393-2.
- Wu et al: Which is the best biologic for nasal polyps: dupilumab, omalizumab, or mepolizumab? A network metaanalysis. Int Arch Allergy Immunol 2022; 183(3): 279-288.
- Boechat et al: Comparing biologicals for severe chronic rhinosinusitis with nasal polyps: a network meta-analysis. Allergy 2022; 77(4): 1299-1306.
Overall, dupilumab performed best in terms of efficacy and safety in these NMAs. The clinical benefit of continued treatment with this biologic was maintained for 24 weeks until the end of follow-up [15]. “This is in line with our experience,” reported the speaker [3]. Real-world studies comparing the different biologics in CRSwNP are few and far between.
Congress: Allergy & Immunology Update
Literature:
- Meier EC, et al.: Real-Life Experience of Monoclonal Antibody Treatments in Chronic Rhinosinusitis with Nasal Polyposis. Int Arch Allergy Immunol 2021; 182(8): 736–743.
- Medix Guideline: Rhinosinusitis, last revised: 09/2021 / last update: 08/2023), www.medix.ch/wissen/guidelines/rhinosinusitis,(last accessed 11.03.2024)
- «The battle against nasal polyps – Where do we stand today?», Prof. Dr. med. Michael Soyka, SYMPOSIUM III: Th2 driven inflammation, Allergy & Immunology Update, 27.01.2024.
- Fokkens WJ, et al.: European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020; 58 (Suppl S29): 1–464.
- Fokkens WJ, et al.: EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology 2023; 61(3): 194–202.
- Hodelin C, et al.: The association of atopic dermatitis and chronic rhinosinusitis in adults: a cross-sectional study using the All of Us research program. Int J Dermatol 2023; 62(8): e430–e431.
- Son DS, Cho MS, Kim DK: Chronic Rhinosinusitis and the Increased Incidence of Atopic Dermatitis. Am J Rhinol Allergy 2022; 36(5): 574–582.
- Simmons JK, et al.: Increased Prevalence of Eosinophilic Esophagitis in Patients With Chronic Rhinosinusitis. Am JRhinol Allergy 2022; 36(6): 804–807.
- Padia R, et al.: Eosinophilic esophagitis strongly linked to chronic rhinosinusitis. Laryngoscope 2016; 126(6): 1279–1283.
- Spezialitätenliste (SL), https://spezialitaetenliste.ch, (last accessed 11.03.2024)
- Bachert C, et al.: Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019; 394(10209): 1638–1650.
- Gevaert P, et al.: Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol 2020; 146(3): 595–605.
- Hopkins C, et al.: Add-on mepolizumab for chronic rhinosinusitis with nasal polyps: SYNAPSE study. Eur Respir J 2020; 56(suppl 64): 4616.
- Bachert C, et al.: Burden of Disease in Chronic Rhinosinusitis with Nasal Polyps. J Asthma Allergy 2021; 14: 127–134. www.ncbi.nlm.nih.gov/pmc/articles/PMC7886239.
- Lou H, Zhang L: Knowledge gaps in using type 2 biologics for real-world treatment of chronic rhinosinusitis with nasal polyps. Allergy 2022; 77(7): 1952–1954.
- Agache I, et al.: Efficacy and safety of treatment with biologicals for severe chronic rhinosinusitis with nasal polyps: a systematic review for the EAACI guidelines. Allergy 2021; 76(8): 2337–2353.
- Oykhman P, et al.: Comparative efficacy and safety of monoclonal antibodies and aspirin desensitization for chronic rhinosinusitis with nasal polyposis: a systematic review and network meta-analysis. J Allergy Clin Immunol 2021; S0091-6749(21):01393-2.
- Wu Q, et al.: Which is the best biologic for nasal polyps: dupilumab, omalizumab, or mepolizumab? A network metaanalysis. Int Arch Allergy Immunol 2022; 183(3): 279–288.
- Boechat JL, et al.: Comparing biologicals for severe chronic rhinosinusitis with nasal polyps: a network meta-analysis. Allergy 2022; 77(4): 1299–1306.
HAUSARZT PRAXIS 2024; 19(3): 38–39 (published on 20.3.24, ahead of print)