Patients with primary advanced or recurrent endometrial cancer have a poor prognosis, particularly in the long-term course of treatment with carboplatin plus paclitaxel. This can be significantly improved with the help of combined immunotherapy. We spoke to PD Dr. Marcus Vetter, Head Physician at the Tumor Center Baselland, about the diagnosis and treatment of endometrial cancer and his experiences with Dostarlimab in clinical practice.
Dr. Vetter, how important is testing for endometrial cancer patients and what should be tested?
Dr. Vetter: The understanding of endometrial cancer has changed significantly in the last 10 years since the discovery of the TCGA data. We have four biological subtypes associated with the new FIGO classification. In any case, a molecular analysis of the most important genes should therefore be carried out, which ultimately classify these four classifications, the POLE mutated, the MSI, the copy number low and copy number high subtypes. Today, these form the basis for our treatment decisions [1,2].
When you think about using Jemperli® – what do you need to bear in mind?
The combination of chemotherapy and dostarlimab has shown great benefit for patients [3]. However, a strong effect is also accompanied by potential side effects. We already know this from chemotherapy alone. It is therefore important to be aware of possible adverse effects, especially during the first six cycles. Lege Artis must undergo regular blood count checks and the chemotherapy and immunotherapy must therefore be regularly reviewed by the treating oncologist. In the case of immunotherapy, special attention must be paid to immune-mediated side effects. These include, for example, hypothyroidism, ulcerative colitis, hepatitis and skin inflammatory reactions. The trained oncologist is very familiar with the side effects of both chemotherapy and immunotherapy.
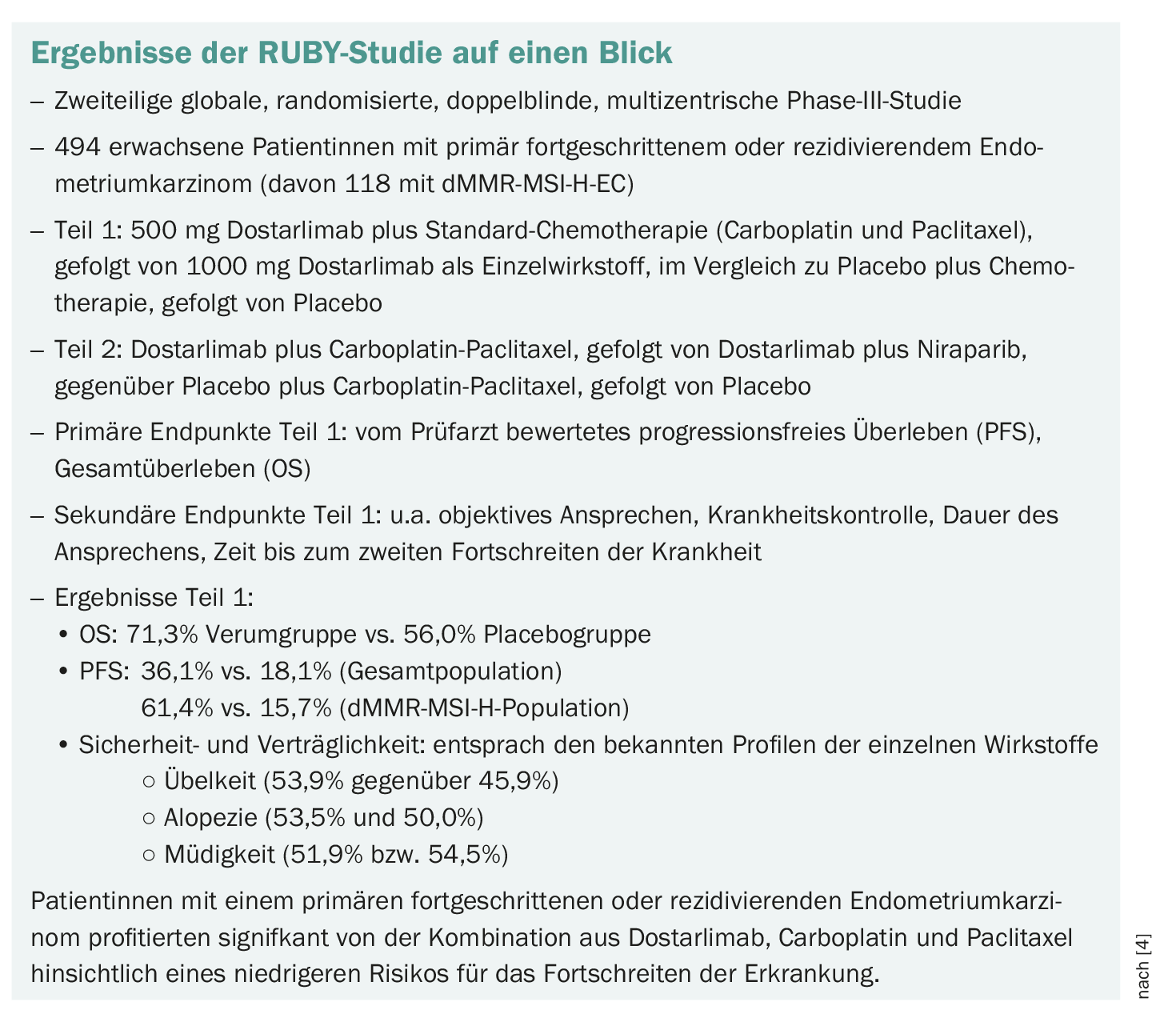
The results of the RUBY study showed significant efficacy and response rate data. What is your experience in everyday clinical practice?
We have been using Dostarlimab since the initial approval and were incredibly pleased when we saw that we had such high response rates. In my opinion, the treatment is relatively well tolerated in everyday life. We were surprised that the so-called MSI patients were progression-free for a very long time and also plateaued in the context of the combination. This was not as pronounced with the non-MSI. Nevertheless, an advantage is also seen in this clientele compared to chemotherapy alone [3–5].
What do you and the nurses observe with regard to the patients’ quality of life? And above all: what do the patients tell you?
Quality of life is particularly important in the case of gynecological tumors, including abdominal tumors. We see very different symptoms in the metastatic stage of endometrial cancer, which also have an impact on quality of life. If the combination therapy responds, we also observe an improvement in the quality of life in many cases. Most patients then suffer from a little fatigue due to the chemotherapy. Hair loss is of course still a medical, partly unsolved problem, which can be very disturbing for patients. But overall, the quality of life is better. This is mainly due to the fact that the therapy is more effective and the response rate is higher [3,4].
Where do you see the advantages and disadvantages of the two studies RUBY and GY-018 – especially with regard to the patient populations in general, the dMMR/MSI-H patient group and the follow-up times?
Both studies incorporated immunotherapy into the treatment, i.e. chemotherapy plus immunotherapy, and came to similar results. Both immune checkpoint inhibitors have shown a clear benefit, especially in the MSI population. These groups had a significantly higher response and longer freedom from progression. No new toxicities were identified. Both studies were relatively comparable [3,4,6].
Patients with distant metastases, but also with localized recurrences, were included in the studies. It is known that patients with stage IIIBC have a very poor prognosis overall. Over 50% of those affected have relapses despite resection and radiotherapy. This relapse rate can be significantly reduced with the new immunotherapy. RUBY is not an adjuvant study. However, it is a study in which a very high-risk collective was examined in some of the patients. It is important to realize that not only stage IV patients but also resected patients with a high risk of relapse benefit from dostarlimab therapy [3,4,6].
What are your thoughts on the efficacy of Dostarlimab + chemotherapy in the different biomarker subgroups?
That is an interesting question. I think that biomarkers will become more and more important. It has been seen with Dostarlimab that biomarkers such as p53 mutations, for example, lead to an improved response. It is important that we measure the microsatellite instability in any case. This will be a very, very good biomarker for us. I think that approval will also come in the area that all MSI patients will receive the drug combinations.
What do the RUBY efficacy data in the various histological subgroups mean for your patients?
I currently have two patients with carcinosarcoma in my consultation. There are patients who have a very reduced prognosis overall. And I’m really happy that I can now offer patients something extra. Although this is only a very rare subgroup, the patients included in the study benefited from the therapy – especially if they had a mismatch repair deficiency [4].
How do you think the therapeutic landscape for endometrial cancer will develop in the future in the first line and later lines?
I think that the immune checkpoint inhibitors are definitely set in the first line. The question is the development in the second line. There is not yet so much good data available. So far, we have only been able to fall back on chemotherapy. I could imagine that the new antibody drug conjugates could play a role here. Then, of course, we will also see data from the PARP inhibitors. And immunotherapy in combination with targeted therapy has certainly not yet been fully exhausted.
Thank you very much for the interview!
The interview was conducted by Tanja Schliebe
Literature:
- Arciuolo D, et al: TCGA Molecular Prognostic Groups of Endometrial Carcinoma: Current Knowledge and Future Perspectives. Int J Mol Sci 2022 Oct 2;23(19): 11684.
- Berek JS, et al: Endometrial Cancer Staging Subcommittee, FIGO Women’s Cancer Committee. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023 Aug; 162(2): 383-394.
- Jemperli® Information for healthcare professionals; status: January 2024. swissmedicinfo.ch
- Mirza MR, et al: Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. Incl. Suppl. N Engl J Med 2023; 388: 2145-2158.
- Oaknin A, et al: Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. Journal for immunotherapy of cancer vol. 10 (2022).
- Ramez N, et al: Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N Engl J Med 2023; 388: 2159-2170.
InFo ONCOLOGY & HEMATOLOGY 2024; 12(1): 30-31