Type 1 diabetes is the most common metabolic disease in children and adolescents. Nowadays, this autoimmune disease can be diagnosed well before clinical manifestation. This enables a “window of opportunity” so that therapeutic intervention can take place before there is a high loss of insulin-producing beta cells and the ketoacidosis caused by metabolic imbalances occurs.
An increase in new cases of diabetes is being observed both internationally and in Switzerland. In the national SOPHYA study, 3 of the 1500 subjects in the 6-16 age group had a diabetes diagnosis, which corresponds to a frequency of 200:100,000 children and adolescents in Switzerland [1]. The incidence of type 1 diabetes (T1D) in Germany, as in many other countries, has risen significantly over the years: while it was 7.9 per 100,000 in the 0-15 age group in 1991-1993, it doubled to 13.3 per 100,000 in 2009-2013 [2]. The early detection of precursors of T1D is a prerequisite for preventive therapeutic measures and the avoidance of potentially life-threatening ketoacidosis. Prof. Dr. med. Michael Hummel from the Helmholtz Zentrum München presented the latest findings on screening and prevention of T1D at this year’s edition of the “Diabetologie grenzenlos” congress [3].
Findings from prospective birth cohort studies
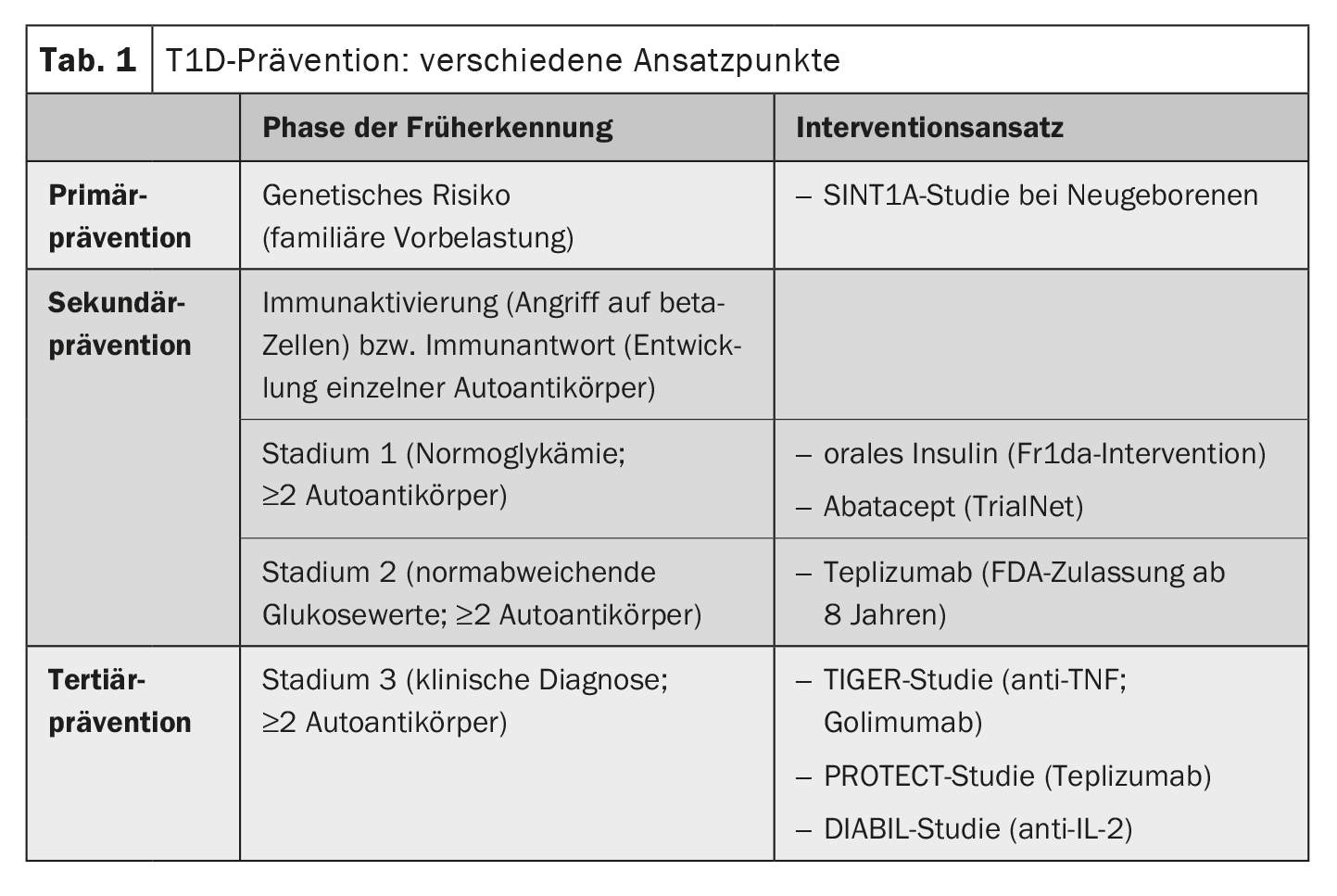
There are numerous different approaches to the prevention of T1D. “There are three potential points in time at which you can intervene,” says Prof. Hummel [3]. These are tertiary prevention, secondary prevention and primary prevention. Most interventions are currently being investigated in studies (Table 1). In the USA, teplizumab has already been approved for the treatment of a preliminary stage of T1D in prediabetic children from the age of 8 [3,4]. Teplizumab is a monoclonal antibody that regulates the immune system [3,4]. This is a milestone that was preceded by years of research. Many years ago, prospective birth cohort studies led to the realization that T1D begins long before the onset of symptoms and that the process of disease development can be detected early by measuring islet autoantibodies in the blood [3]. The detection of islet autoantibodies in combination with other metabolic markers makes it possible to predict the progression of the autoimmune disease and the probable time of manifest symptoms [5]. Data from the prospective birth cohort studies BABYDIAB and TEDDY show that islet autoantibodies most frequently appear in the blood for the first time between the ages of 9 months and 2 years [5]. If two or more of the antibodies can be detected, there is an almost 100% probability of T1D, so that an intervention at this point seems sensible and justified [3]. If the blood glucose values are normal in this constellation, this is referred to as stage 1 of T1D. If the disease process is already more advanced and slightly elevated blood glucose levels can also be measured, this is referred to as stage 2. Stage 3 corresponds to the classic manifestation of T1D with clinical symptoms and potentially life-threatening ketoacidosis; at this point, around 80% of the beta cells have already been destroyed.
Secondary prevention: Teplizumab delays T1D manifestation
The monoclonal anti-CD3 antibody teplizumab was the first preventive, immunomodulatory therapy to be approved by the US Food and Drug Administration (FDA) in 2022 – with the status of a “breakthrough” drug (box) [3]. Treatment with teplizumab is currently approved in the USA for prediabetic children aged 8 years and older. “It is the first substance to succeed in delaying the onset of diabetes by around three years,” explained Prof. Hummel [3]. The mechanism of action of teplizumab is that the substance is directed against activated T lymphocytes that have the surface marker CD3 [3]. This also suppresses those autoreactive T cells that are directed against the beta cells. At the same time, the relative proportion of regulatory T cells increases. Teplizumab is used in people with early stage 2 (at least two positive islet autoantibodies and first, early signs of impaired glucose metabolism). A 14-day intravenous therapy cycle with teplizumab delays the clinical manifestation of T1D by an average of almost three years and stabilizes residual beta cell function [3,6].
Although teplizumab therapy can only delay the progression of the disease, it cannot stop it completely, i.e. it does not prevent the manifestation of T1D. However, delaying the progression of diabetes in children is clinically significant in terms of outcomes and disease management in everyday life [6]. And in the long term, better residual beta cell function, characterized by higher C-peptide levels, is associated with improved blood glucose control. The most common side effects of teplizumab that occurred transiently during the treatment phase included transient lymphocytopenia, rash and headache [6]. The side effects are not dramatic, summarized Prof. Hummel. No serious side effects have occurred to date [4]. The immunotherapeutic agent is also expected to be approved for Europe. Against this background, it is clear that population-based screening programs are needed to identify children who can benefit from treatment in good time [3].
Primary prevention: ongoing studies
Efforts are being made even earlier to take primary preventive action before antibodies appear. This involves treating children in whom a screening a few days after birth indicates a high genetic risk of T1D. In Bavaria, this screening is carried out within the GPPAD consortium; over 500,000 children from the general population have already been tested [3,8,9]. “There will probably never be a single primary prevention measure that prevents all cases of type 1 diabetes, as different individual environmental factors are involved,” says Prof. Hummel [3]. At present, primary preventive therapies are mainly offered within the framework of studies. Children with a significantly increased risk of T1D are treated with oral insulin (POInt study) or probiotics (SINT1A) [7]. Specifically, the SINT1A study is investigating whether the administration of the probiotic B. infantis can prevent the development of T1D in high-risk patients by reducing dysbiosis and inflammation in the gut [8]. In the POInT study, daily administration of insulin powder together with a meal is used to train the immune system in such a way that no incorrect immune response occurs [9]. The recruitment phase of the POInT study has now been completed, over 1000 participants have been included and the evaluation is expected in 2025. More than 1000 participants have already been recruited for the SINT1A study. Numerous research efforts are therefore underway to make the goal of delaying or preventing the manifestation of T1D with early interventions more achievable. Fortunately, the treatment options for manifest T1D are also constantly improving, particularly thanks to diabetes technology innovations such as continuous glucose monitoring and insulin pumps [3]. The consistent treatment of concomitant and secondary diseases such as lipometabolic disorders and kidney damage also significantly improves the outcome of those affected. The speaker points out that people with T1D still have a reduced life expectancy compared to the general population, especially if they fall ill before the age of 10, as Swedish registry data show [3,10]. But thanks to modern treatment options, the conditions are constantly improving.
Congress: Diabetologie grenzenlos
Literature:
- Bringolf-Isler B, et al: Final report on the SOPHYA study. Basel: Swiss Tropical and Public Health Institute, 2016, www.bag.admin.ch/dam/bag/de/dokumente/npp/forschungsberichte,(last accessed 23.02.2024)
- Patterson CC, et al: Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centers in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia 2019; 62(3): 408-417.
- “Prevention of type 1 diabetes: (immune) interventions”, Prof. Dr. med. Michael Hummel, Diabetologie grenzenlos, Munich, 02.-03.02.2024.
- Ramos EL, et al; PROTECT Study Investigators. Teplizumab and β-Cell Function in Newly Diagnosed Type 1 Diabetes. N Engl J Med 2023; 389(23): 2151-2161.
- Ziegler AG: The countdown to type 1 diabetes: when, how and why does the clock start? Diabetologia 2023;66(7): 1169-1178.
- Herold KC, et al; Type 1 Diabetes TrialNet Study Group. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med 2019; 381(7): 603-613.
- Ziegler AG, et al: GPPAD STUDY GROUP. Supplementation with Bifidobacterium longum subspecies infantis EVC001 for mitigation of type 1 diabetes autoimmunity: the GPPAD-SINT1A randomized controlled trial protocol. BMJ Open 2021; 11(11): e052449.
- SINT1A study: Prevention of type 1 diabetes, www.gppad.org,(last accessed 23.02.2024)
- POInT study: Prevention of type 1 diabetes, www.gppad.org,(last accessed 23.02.2024)
- Rawshani A, et al: Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 2018; 392(10146): 477-486.
HAUSARZT PRAXIS 2024; 19(3): 50-52 (published on 20.3.24, ahead of print)