- Long-term treatment with upadacitinib (UPA, RINVOQ®) for up to 5.5 years shows no new safety signals [1].
- UPA has a consistent safety profile in the treatment of atopic dermatitis (AD), rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (AS), although the incidence of adverse events varies due to differences in the patient population and disease-related comorbidities[1].
UPA in atopic dermatitis
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease worldwide and places a considerable burden on the lives of patients and their families [2]. In addition, AD is associated with various allergic, autoimmune and cardiovascular comorbidities [3]. For patients with moderate to severe AD who are refractory to topical treatment, there are targeted systemic therapies that reduce relapses and also mitigate the psychological effects of the disease [1, 4-6]. UPA is an oral, reversible Janus kinase (JAK) inhibitor that acts specifically on JAK1 and to a lesser extent also on JAK2, JAK3 or TYK2. UPA is used in a dosage of 15 mg once daily for the treatment of AD, RA, PsA and AS [7].
UPA showed strong efficacy in all 12 studies in AD, RA, PsA and AS. However, safe use is equally crucial for treatment [7]. The data from the ORAL surveillance study, which compared the JAK inhibitor tofacitinib with a tumor necrosis factor (TNF) inhibitor in elderly patients with RA and cardiovascular risk factors, underscores the need to better characterize the safety profile of JAK inhibitors – in part because the safety of individual JAK inhibitors varies among different patient groups with immune-mediated inflammatory diseases (IMIDs) [1]. A new publication by Burmester et al. now shows the long-term safety profile of UPA over a period of up to 5.5 years in various rheumatic diseases and up to 2.75 years in AD. No new safety signals occurred during this period [1].
Long-term treatment with UPA
In total, the safety of UPA was investigated in 6000 patients with AD, RA, PsA and AS with over 15,000 patient years (PY). The safety data from 12 Phase IIb/III studies for each disease were pooled across all studies. Three of these studies with a total of 2693 study participants were conducted in AD. This corresponds to 2035.8 PY.
Table 1: Basic demographic data and disease characteristics of UPA in AD patients
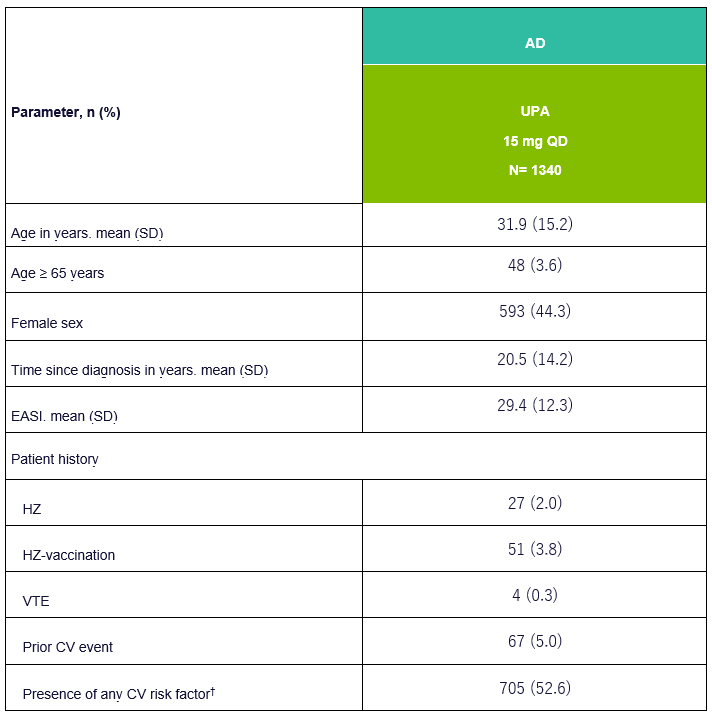
EASI, Eczema Area and Severity Index; VTE, Venous Thromboembolism; CV, Cardiovascular; HZ, Herpes Zoster; †CV risk factors include history of cardiovascular events, hypertension, diabetes mellitus, tobacco/nicotine use, elevated LDL-C and decreased HDL-C. Adapted from [1]
No new safety signals under UPA in the AD
Serious infections under UPA 15 mg occurred in AD at a rate of 2.5 events per 100 PY (2.5 E/100 PY) and rarely led to discontinuation of treatment. The most common serious infection in all populations surveyed was COVID-19, followed by herpes zoster in the AD population. This was generally mild or moderate, without involvement of the central nervous system or internal organs, rarely led to interruption of therapy and usually only affected a single dermatome. In addition, no association between the duration of treatment and the incidence of herpes zoster was found [1]. Vaccination against herpes zoster is available. The vaccine should be administered 4 weeks before treatment with an active immunomodulatory agent such as UPA [7]. Other opportunistic infections under UPA 15 mg were listed as 1.8 events per 100 PY, of which mainly eczema herpeticum was reported. Tuberculosis (TB) was recorded separately and active TB was recorded at 0.03 E/100 PY in AD patients under UPA. The most common treatment-emergent adverse event (TEAE) associated with UPA was an upper respiratory tract infection [1].
Majoradverse cardiovascular events (MACE) included the following Death, non-fatal myocardial infarction and non-fatal apoplexy. You have been reported in AD patients at a rate of 0.03/100 PY. No correlation was found between the duration of UPA intake and the occurrence of MACE. Furthermore, most patients who suffered a MACE had at least one cardiovascular risk factor [1].
Venous thromboembolism (VTE ) included deep vein thrombosis and pulmonary embolism. They occurred at a rate of 0.04 E/100 PY, with most patients having at least one cardiovascular and/or thromboembolic risk factor. No correlation was found between the duration of exposure to UPA and the occurrence of VTE [1].
Malignant diseases (excluding non-melanoma skin cancer, NMSC) were registered under UPA 15 mg at 0.2 E/100 PY. No significant change in this rate was seen throughout the duration of UPA use. The NMSC events were generally not severe and did not lead to discontinuation of treatment. NMSC were reported in the UPA 15 mg population at 0.3 E/100 PY [1].
Laboratory anomalies
Dose-dependent side effects such as anemia, neutropenia, lymphopenia, liver dysfunction and creatine phosphokinase (CPK) elevation have also been reported in AD patients taking UPA. Most laboratory abnormalities were classified as overall mild and transient and only a few led to discontinuation of study medication [1].
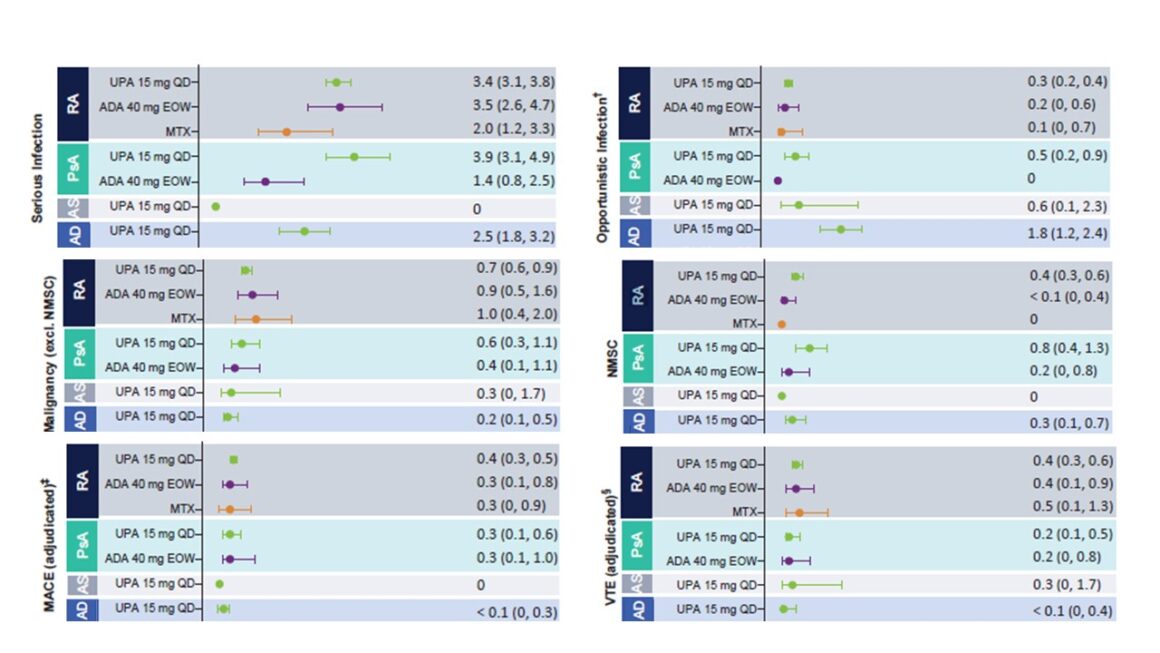
Figure 1: Exposure-adjusted rates of treatment-emergent adverse events (TEAEs) of particular interest. Adapted from [1]
This safety analysis shows no new or unexpected safety risks under UPA compared to previous reports. An increased risk of herpes zoster and increased CPK levels are associated with JAK inhibition and are consistent with the general safety profile of JAK inhibitors. Herpes zoster infections were mostly non-severe and limited to one dermatome, while elevated CPK levels were mostly asymptomatic and transient. Although VTE events were reported in patients receiving UPA, the rates were consistent with the background rates for the individual conditions. [1].
Conclusion
In summary, UPA was generally well tolerated. The safety profile is comparable for AD, RA, PsA and AS, although the incidence of adverse events varies due to differences in the patient population and disease-related comorbidities. Even with long-term treatment with UPA of up to 5.5 years or 2.75 years in AD, no new safety signals were detected.
The full publication by Burmester et al. can be found here.
JAK, Janus Kinase; TYK, Tyrosine Kinase; IMIDs, Immune-Mediated Inflammatory Diseases; EASI, Eczema Area and Severity Index; HZ, Herpes Zoster; MACE, Major Adverse Cardiovascular Event; NMSC, Non-Melanoma Skin Cancer; UPA, Upadacitinib; VTE, Venous Thromboembolism; TEAEs, Treatment-Emergent Adverse Events; LDL-C, Low Density Lipoprotein-Cholesterol; HDL-C, High Density Lipoprotein-Cholesterol; CPK, Creatine Phosphokinase.
References:
1. Burmester, G.R., et al, Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open, 2023. 9(1).
2 Ali, F., J. Vyas, and A.Y. Finlay, Counting the Burden: Atopic Dermatitis and Health-related Quality of Life. Acta Derm Venereol, 2020. 100(12): p. adv00161.
3 Langan, S.M., A.D. Irvine, and S. Weidinger, Atopic dermatitis. Lancet, 2020. 396(10247): p. 345-360.
4 Song, A., S.E. Lee, and J.H. Kim, Immunopathology and Immunotherapy of Inflammatory Skin Diseases.Immune Netw, 2022. 22(1): p. e7.
5 Silvestre JF et al. Real-World Burden in Patients With Atopic Dermatitis Who Are Candidates for Systemic Therapy and Currently Receiving No Systemic Therapy, No Treatment, Topical Therapy Alone, or Systemic Therapy: Results From a Real-World Multicountry Study. EADV, Milan, September 7-11, 2022. Oral session FC02.02.
6 Megna, M., et al, Systemic Treatment of Adult Atopic Dermatitis: A Review. Dermatol Ther (Heidelb), 2017. 7(1): p. 1-23.
7. current technical information RINVOQ® (upadacitinib) at www.swissmedicinfo.ch
The references can be requested by professionals at medinfo.ch@abbvie.com.
Author: Dr. med. Corinne Peter
Brief technical information RINVOQ®
This article was produced with the financial support of AbbVie AG, Alte Steinhauserstrasse 14, Cham.
CH-RNQD230038_11/2023
Contribution online since 23.10.2023
This article was released in German
Post updated: 11/17/2023












