Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiomyopathy worldwide, affecting around 1 in 500 people. Current therapeutic interventions include lifestyle optimization, medications, septal reduction therapies and, rarely, heart transplantation. Advances in the understanding of disease-causing genetic variants in HCM and their molecular mechanisms have opened up the potential for targeted therapies and the implementation of precision and personalized medicine. Results from preclinical research are promising and raise the question of whether a cure for some HCM subtypes may be possible in the future.
Hypertrophic cardiomyopathy (HCM) is a primary myocardial disease characterized by unexplained left ventricular hypertrophy that occurs in the absence of abnormal stress conditions such as hypertension or aortic stenosis. The clinical presentation of HCM is highly variable, ranging from asymptomatic individuals to those with severe symptoms such as heart failure, arrhythmias and sudden cardiac death (SCD). The genetic basis of HCM is also heterogeneous, with mutations in at least eight sarcomere genes identified as pathogenic and a significant proportion of cases associated with genetic variants of undetermined significance (VUS).
Current therapy landscape for HCM
Lifestyle measures: Lifestyle optimization is critical to the management of HCM. Avoiding uncontrolled hypertension and obesity is essential to prevent exacerbation of the HCM phenotype. Recent studies have also emphasized the benefits of regular, light to moderate exercise in improving quality of life and cardiovascular outcomes in HCM patients. Previously, HCM patients were cautioned against intense physical activity, but recent research suggests that moderate exercise can be safe and beneficial.
Drug therapy: Drugs such as beta blockers and calcium channel blockers are often used to relieve symptoms and reduce the risk of complications of HCM. These drugs work by lowering the heart rate and reducing the contractility of the heart, which helps to reduce left ventricular outflow tract obstruction. Newer therapies such as mavacamten, a myosin inhibitor, have shown promising results in recent studies, improving subjective and objective symptoms in patients with obstructive HCM. Mavacamten directly modifies the β-myosin heavy chains to reduce the affinity between actin and myosin and thus normalize cardiac function.
Interventional therapies: Interventional procedures, including septal myectomy and alcohol septal ablation, are performed to relieve left ventricular outflow tract obstruction (LVOT). Septal myectomy is a surgical intervention in which a portion of the thickened septum is removed to improve blood flow from the left ventricle. This method is typically used in younger patients. Alcohol septal ablation, a less invasive alternative, is more commonly performed in older patients. It involves injecting alcohol into the septal arteries to cause controlled infarction and reduce hypertrophy. Despite higher rates of arrhythmias and scarring compared to septal myectomy, alcohol septal ablation can achieve excellent results in experienced centers.
Prevention of sudden cardiac death: Implantable cardioverter defibrillators (ICDs) are recommended for people at high risk of SCD. Advances in risk stratification and the availability of ICDs have significantly reduced HCM-related mortality. Risk assessment is performed using guideline-based risk calculators that take into account factors such as septal thickness, the presence of nonsustained ventricular tachycardia, and family history.
Heart transplant: In rare cases, when patients fail to respond to all other treatments and develop advanced heart failure, a heart transplant may be necessary. These patients make up about 1.6% of those awaiting a heart transplant in the US. Despite its rarity, the survival rate after heart transplantation for HCM patients is similar to that of patients with other cardiomyopathies.
Gene therapy – the new frontier?
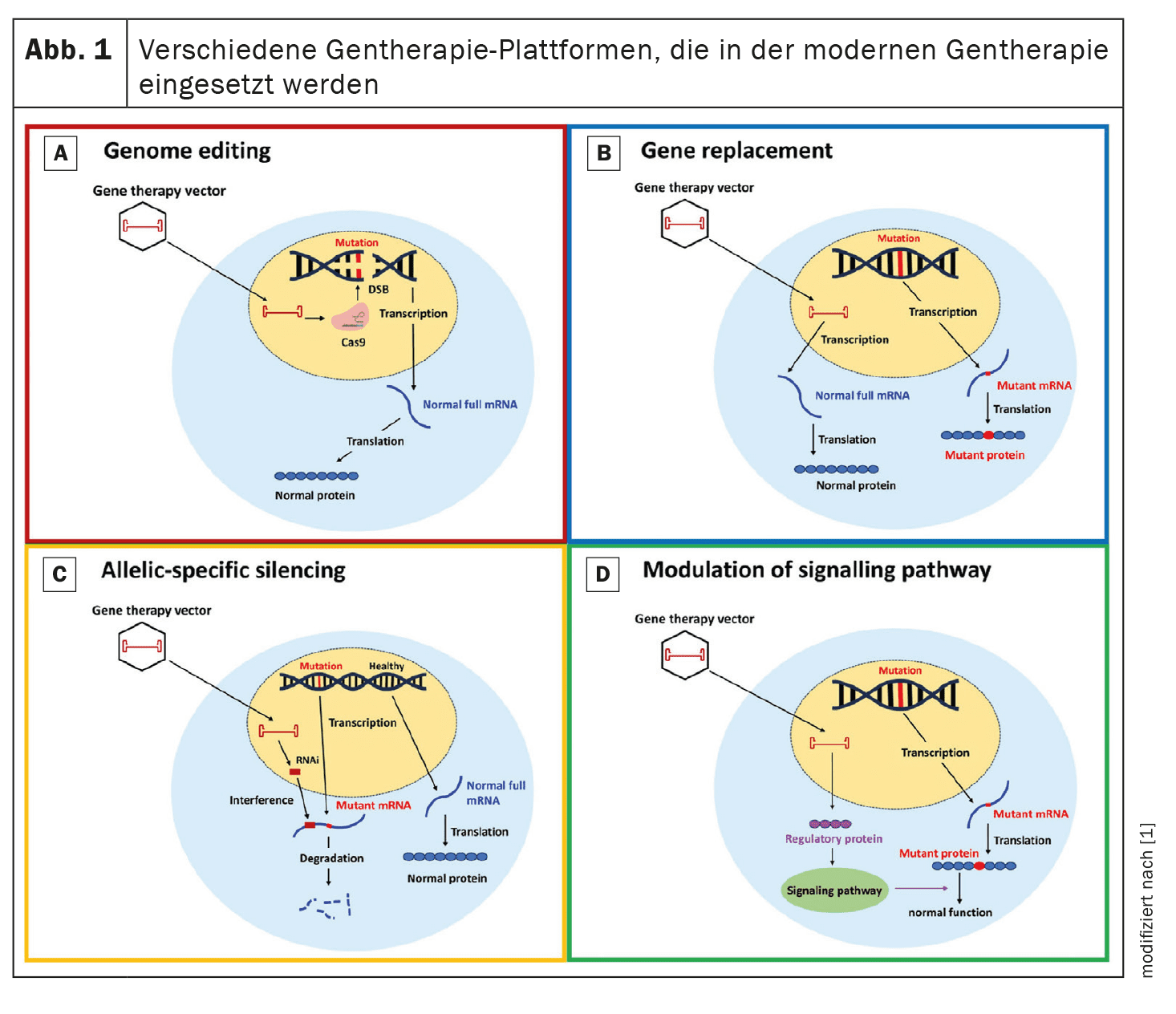
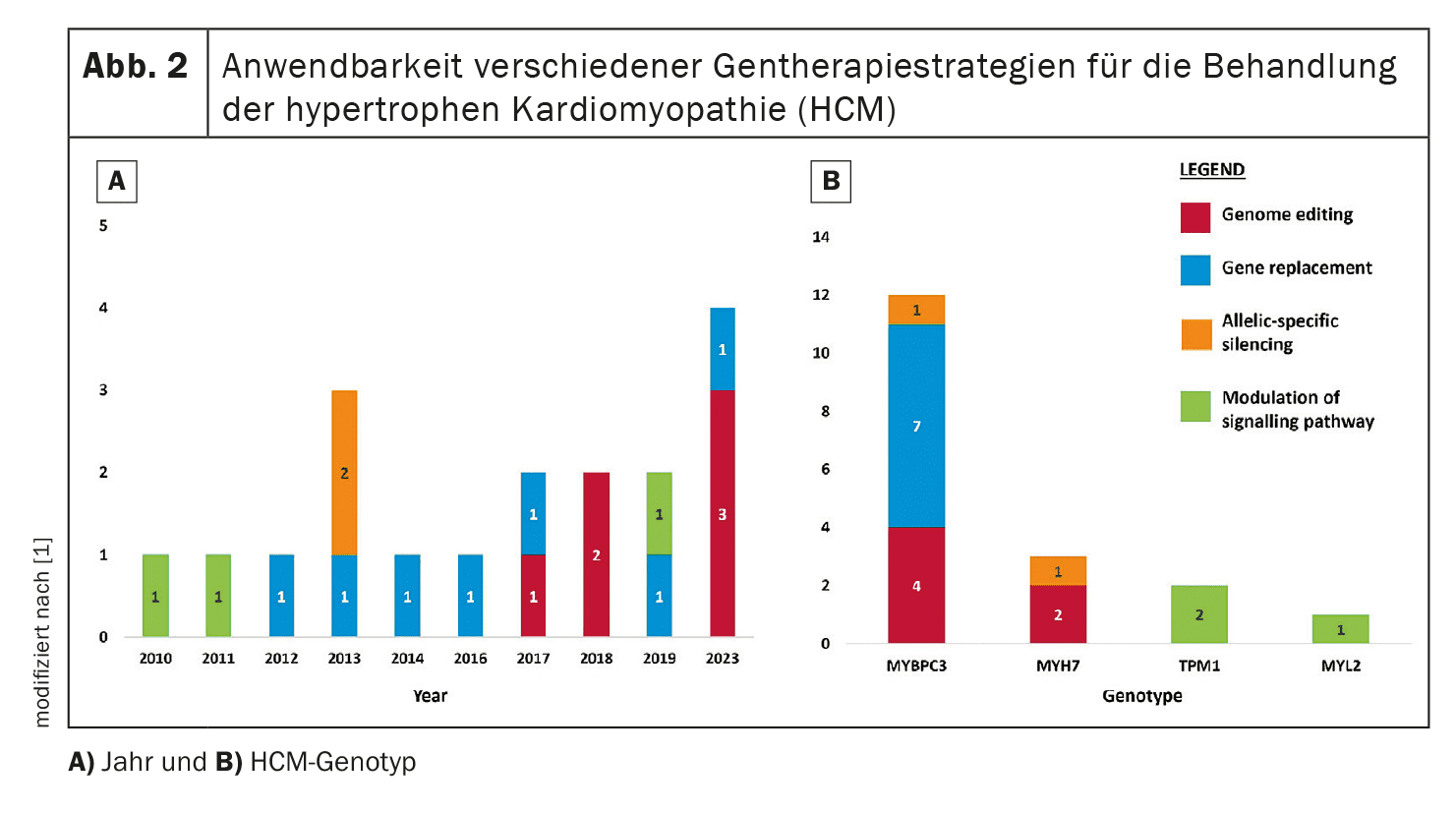
Gene therapy aims to correct or attenuate the genetic mutations responsible for HCM. Four main approaches are examined in this review: Genome editing, gene replacement, allele-specific silencing and pathway modulation.
Genome editing: Genome editing with CRISPR/Cas9 technology has shown the potential to correct HCM-associated genetic mutations in preclinical models.
CRISPR/Cas9 uses a programmable nuclease that creates targeted DNA double-strand breaks that can be repaired by non-homologous end-joining (NHEJ) or homologous recombination (HDR).
However, challenges such as off-target effects and the need for precise delivery methods remain significant hurdles.
Research is focused on improving the specificity and efficiency of this technology to minimize unintended genetic alterations.
Gene replacement: Gene replacement therapy involves the introduction of a functional copy of the mutated gene.
This approach is particularly relevant for mutations leading to haploinsufficiency.
Ongoing studies, such as the use of adeno-associated virus (AAV) vectors, aim to evaluate the efficacy and safety of this strategy in HCM patients.
For example, gene replacement of the MYBPC3 gene, which is frequently mutated in HCM, is currently being investigated in clinical trials.
In preclinical studies, replacement of the defective gene has been shown to improve myocardial function and prevent HCM phenotypes.
Allele-specific silencing: Allele-specific silencing aims to suppress the mutant allele while preserving the function of the normal allele. This approach utilizes small interfering RNAs (siRNAs) to selectively degrade the mutant mRNA and reduce the production of abnormal proteins. This method is particularly useful for dominant mutations where the mutant protein has a deleterious effect. Preclinical studies have shown that siRNAs can effectively reduce the expression of the mutant allele, resulting in improved cardiac function and reduced hypertrophy.
Modulation of signaling pathways: Modulation of key signaling pathways involved in the pathogenesis of HCM offers another therapeutic approach. For example, increasing the expression of SERCA2a, a protein involved in calcium balance, has shown potential in preclinical models to reduce hypertrophy and improve cardiac function. Another approach involves modulating the phosphorylation of the myosin regulatory light chain (myosin RLC) to improve cardiac contractile function.
Challenges and future directions
Despite promising progress, there are several challenges that hinder the clinical translation of gene therapies for HCM. These include:
- Safety concerns: Risks associated with AAV vectors, such as immune responses and off-target effects, need to be thoroughly addressed. Recent deaths associated with AAV-based gene therapies are a reminder of the potential dangers and the need to further improve the safety of these technologies.
- Delivery methods: Efficient and targeted delivery of gene therapies to cardiac tissue remains a technical challenge. The development of vectors with specific and improved cardiac tropism could help to minimize off-target transductions and reduce the required doses.
- Genetic heterogeneity: The genetic diversity of HCM complicates the development of universal gene therapy approaches. Since HCM is caused by a variety of mutations, the development of specific gRNAs for each mutation and the limitation by PAM sequences requires further research and optimization.
- Ethics and equitable access: Ensuring that gene therapies are accessible to all patients, regardless of socio-economic status, is crucial. Equitable distribution of these advanced treatments must be a priority to avoid health inequalities.
Conclusion
Advances in gene therapy hold great promise for the future treatment of HCM. While preclinical research has shown potential, translating these therapies into clinical practice will require significant scientific, technical and ethical challenges. Equitable access to these advanced treatments must be ensured to realize their full potential to improve outcomes for HCM patients. Further research and development of safe and effective gene therapies could revolutionize the way HCM and other genetic cardiomyopathies are treated.
Source:
- Paratz ED, Mundisugih J, Rowe SJ, et al.: Gene Therapy in Cardiology: Is a Cure for Hypertrophic Cardiomyopathy on the Horizon? Can J Cardiol 2024 May; 40(5): 777–788. doi: 10.1016/j.cjca.2023.11.024. Epub 2023 Nov 25. PMID: 38013066.
CARDIOVASC 2024; 23(2): 24–25