Numerous studies published in the last two years made it necessary to update the 2021 ESC Heart Failure Guidelines. In particular, this refers to new studies on SGLT2 inhibitors in the area of HFpEF and HFmrEF, intravenous iron therapy and treatment after cardiac decompensation.
In cardiology in particular, the shelf life of guideline recommendations is often limited due to the ongoing intensive research activities. This also applies to the guidelines for the management of heart failure. After the European Society of Cardiology (ESC) had just updated its European Heart Failure Guidelines 2021 to the latest scientific standards, cardiology research quickly followed suit with more than a dozen new and practice-relevant clinical studies. Some of these now shape the new recommendations in the 2023 focused update.
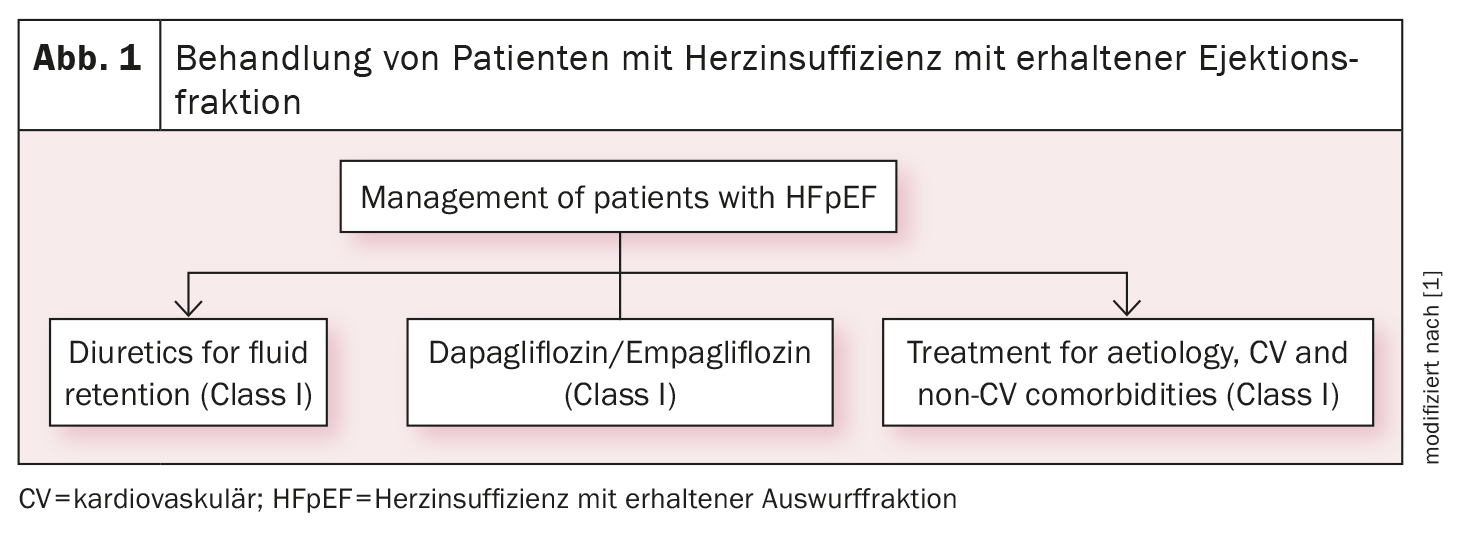
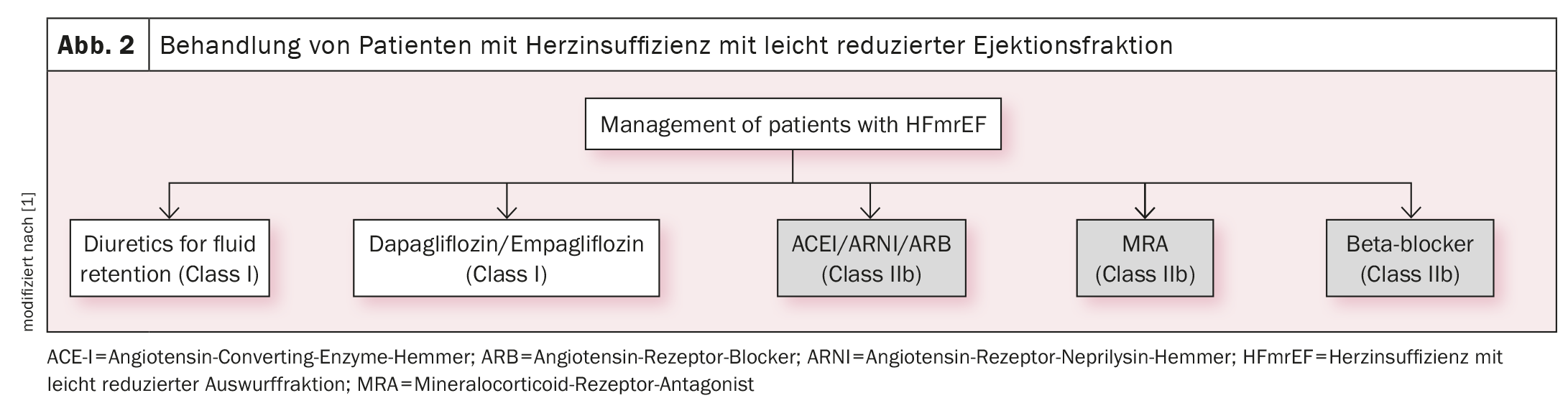
Recommendation for SGLT2 inhibitors in HFmrEF and HFpEF
Based on the EMPEROR-Preserved and DELIVER studies, the SGLT2 inhibitors empagliflozin and dapagliflozin now have a class IA indication (in addition to diuretics) for the treatment of heart failure with preserved (HFpEF, LVEF ≥50%) (Fig. 1) [1] or mildly reduced left ventricular function (HFmrEF, LVEF 41%-49%) (Fig. 2) [1]. There are now two identical strong recommendations for both heart failure phenotypes: “An SGLT2 inhibitor (dapagliflozin or empagliflozin) is recommended in patients with HFpEF/HFmrEF to reduce the risk of hospitalization for heart failure or cardiovascular death”.
Management strategies for decompensated heart failure
The 2021 guideline already recommended an intensive strategy of early initiation (if possible before discharge) and rapid uptitration of evidence-based treatment approaches (“fantastic four”), albeit as an expert consensus. In the current update, based on the STRONG-HF study, this strategy now has a class IB indication for reducing the risk of heart failure rehospitalization or death.
Early and rapid initiation of optimized heart failure therapy
There are also new recommendations for the management of acute heart failure. These relate primarily to discharge management. Evidence-based heart failure medication should still be prescribed and titrated in hospital. After discharge from hospital, the medication should be reviewed within six weeks and adjusted if necessary. This recommendation is based on the results of the STRONG-HF study, in which rapid and closely monitored initiation of guideline-compliant medication in hospitalized patients with acute heart failure (mostly HFrEF) significantly reduced the incidence rate of death and readmission for heart failure (primary combined endpoint) compared to usual routine treatment.
Titration in STRONG HF must have taken place within 14 days of hospital discharge. This very tight time frame has been extended to six weeks in the new ESC guidelines. During post-discharge checks, particular attention should be paid to symptoms and signs of congestion, blood pressure, heart rate, NTproBNP values, potassium concentration and renal function (eGRF).
New recommendations for heart failure prevention in kidney disease
There are also new recommendations with regard to comorbidities and the prevention of heart failure in special risk groups, such as patients with chronic kidney disease and type 2 diabetes. The focus is on two pharmacotherapies, SGLT2 inhibitors and the mineral corticoid receptor antagonist (MRA) finerenone. They are recommended for people with diabetes mellitus and/or chronic kidney disease to prevent hospitalization for heart failure and cardiovascular death: “In patients with type 2 diabetes and chronic kidney disease, SGLT2 inhibitors (dapagliflozin or empagliflozin) are recommended to reduce the risk of hospitalization for heart failure or cardiovascular death” and “In patients with type 2 diabetes and chronic kidney disease, finerenone is recommended to reduce the risk of hospitalization for heart failure.”
The recommendations made regarding SGLT2 inhibitors have their scientific evidence in the two studies DAPA-CKD (with dapagliflozin) and EMPA-KIDNEY (with empagliflozin). Both studies showed that SGLT2 inhibition not only significantly slowed the progression of renal disease, but also reduced the risk of the combined endpoint of hospital admissions for heart failure and cardiovascular death.
According to a meta-analysis of four SGLT2 inhibitor studies (DAPA-CKD, EMPA-KIDNEY, CREDENCE, SCORED), the reduction in cardiovascular events in kidney patients with and without type 2 diabetes was comparable, but not significant in non-diabetics. SGLT2 inhibitors are therefore only recommended for heart failure prevention in patients with chronic kidney disease and type 2 diabetes.
The recommendation of finerenone for patients with type 2 diabetes and chronic kidney disease is based on the FIDELIO-DKD and FIGARO-DKD studies and an analysis of their pooled data (FIDELITY). According to the study, finerenone not only reduced the progression of diabetic nephropathy, but also the risk of a combined cardiovascular endpoint (cardiovascular death, myocardial infarction, stroke, hospitalization). With regard to the endpoint components, the 22% reduction in hospitalizations for heart failure also proved to be significant.
Intravenous iron supplementation recommended for symptom relief
Other changes concern the complex issue of iron supplementation in patients with heart failure and iron deficiency. This is one of the most common non-cardiovascular comorbidities in the heart failure population. Iron deficiency has proven to be a very unfavorable prognostic factor and is also associated with a very poor quality of life. A significant improvement in function and quality of life through intravenous iron supplementation has been demonstrated in several studies. Therefore, the ESC guideline update contains a strong recommendation for iron supplementation in patients with HFrEF and HFmrEF and iron deficiency, with the aim of improving symptoms and quality of life, but only a IIa recommendation regarding the reduction of hospitalizations: “Intravenous iron supplementation is recommended in symptomatic patients with HFrEF and HFmrEF to relieve heart failure symptoms and improve quality of life” and “Intravenous iron supplementation with ferric carboxymaltose or ferric derisomaltose should be considered in symptomatic patients with HFrEF and HFmrEF and iron deficiency to reduce the risk of heart failure hospitalizations.”
The second recommendation is based on the results of the AFFIRM-AHF and IRONMAN studies, in which the incidence of hospital admissions due to heart failure was reduced by iron supplementation – albeit not significantly.
Source:
- McDonagh TA, et al: 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal, Volume 44, Issue 37, October 1, 2023, Pages 3627-3639, https://doi.org/10.1093/eurheartj/ehad195.
CARDIOVASC 2023; 22(4): 36-37 (published on 28.11.23, ahead of print)