Diagnosis at an early stage of the disease is a prerequisite to take advantage of the time window for optimally effective disease-modifying therapy (DMARD). This not only enables a reduction in pain and inflammation, but can also halt progressive joint destruction and associated functional limitations. Disease activity can be objectified using the DAS28 score.
Rheumatoid arthritis (RA) incidence rates are highest in the 55- to 75-year-old age groups [1,2]. Characteristic features of this inflammatory rheumatic disease include symmetrical metacarpophalangeal and peripheral interphalangeal joint involvement, with early stage metatarsophalangeal joints also affected. The inflammation of the joints underlying RA not only leads to pain, swelling, and functional limitations, but can also result in progressive joint destruction if inadequately treated [3]. Inflammation is a determinant of outcome in terms of preservation of physical function as well as comorbidities and mortality [4].
“Don’t miss “Windows of opportunity
It is considered empirically proven that rapid control of disease activity by early initiation of therapy with disease-modifying agents is crucial for further prognosis [3]. The S3 guidelines updated in 2019 therefore suggest initiating disease-modifying therapy (DMARD) in the presence of RA within three months of symptom onset. Coordinated interdisciplinary care is a prerequisite for diagnosis at the earliest possible stage, ensuring that the indication for disease-modifying therapy is assessed and that affected individuals are referred to a rheumatologist promptly [4]. The ACR/EULAR classification criteria are intended to promote the earliest possible initiation of disease-modifying therapy and the prevention of irreversible disease sequelae [5,6,14] . Based on certain criteria, a sum score is calculated (Tab. 1). With a score of 6 or more, the diagnosis of rheumatoid arthritis can be made. Common clinical manifestations of RA include morning stiffness, tendon crepitation, and interosseous atrophy [7]. Laboratory findings include a positive rheumatoid factor and ACPA. In the current S3 guideline, a diagnostic algorithm is proposed to facilitate RA diagnosis. “Typical of arthritis is a palpable, soft, ‘elastic’ swelling of a joint caused by effusion and/or inflammatory thickening of the synovium (synovitis, synovialitis), accompanied by pain and joint stiffness,” reads a diagnostic tip for differential diagnosis according to the S3 guideline [3,4].
“Treat-to-target” using DMARDs.
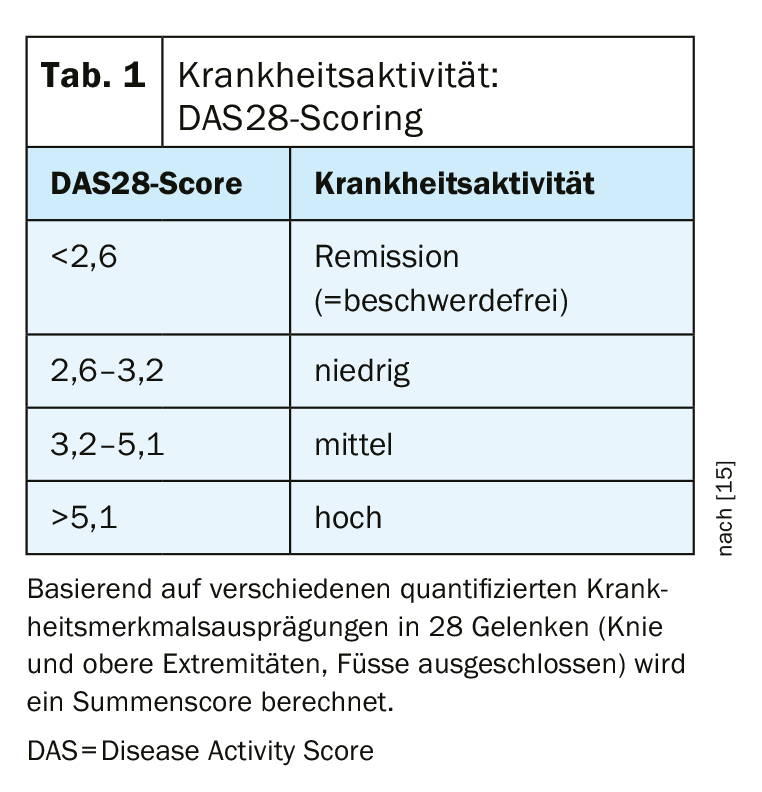
The DAS28 scoring system (DAS=Disease Activity), which serves to assess disease activity according to certain clinical and biological parameters, is used to objectify the success of therapy [15]. Based on quantified disease characteristics in 28 joints (knees and upper extremities, feet excluded), a sum score is calculated, whereby a value <2.6 is considered a criterion for remission (Tab. 1) . It has been repeatedly proven that limitations in terms of physical and mental health correlate positively with high disease activity [6,14]. It also showed that the risk of joint replacement surgery was associated with the extent of disease activity. In addition, it became clear that in the long-term course, the risk for joint replacement surgery correlates positively with the extent of disease activity. Disease-modifying anti-rheumatic drugs (DMARDs), can slow the progression of rheumatoid arthritis by intervening in the disease process. In addition to biologics and biosimilars (bDMARDs) and conventional synthetic substances (csDMARDs), the disease-modifying antirheumatic therapeutics also include the targeted synthetic agents (tsDMARDS), to which the Janus kinase inhibitors are assigned. In contrast to biologics, which inhibit certain extracellular messenger substances of the immune system, such as TNF or interleukins, the mode of action of Janus kinase inhibitors (JAK inhibitors) is based on an interruption of the intracellular signal transduction of Janus kinases. This produces an anti-inflammatory effect. In January 2020, upadacitinib (Rinvoq®) was approved in Switzerland for the first time as a JAK inhibitor for the treatment of rheumatoid arthritis [12,13].
Literature:
- Zink A, Albrecht K: Zeitschrift fur Rheumatologie 2016;75: 346-353.
- Hense S, et al: Zeitschrift fur Rheumatologie 2016;75: 819-827.
- Schneider M, et al: Interdisciplinary guideline management of early rheumatoid arthritis. AWMF 060/002, S3, 4th revised and expanded edition, 2019.
- Combe B, et al: Annals of the rheumatic diseases 2017; 76: 948-959.
- Aletaha D, et al: Annals of the rheumatic diseases 2010;69: 1580-1588.
- Radner H, et al: Remission in rheumatoid arthritis: benefit over low disease activity in patient-reported outcomes and costs. Arthritis Research & Therapy 2014; 16: Article number: R56.
- Erni S: DD of Rheumatology, Stephan Erni, MD, RehaClinic AG, Bad Zurzach, slide presentation, FOMF Basel, Jan. 30, 2020.
- Lindqvist E, et al: Annals of the rheumatic diseases 2002; 61: 1055-1059.
- van Zeben D, et al: The Journal of rheumatology 1994; 21: 1620-1625.
- Wiles NJ, et al. Arthritis and rheumatism 2001; 44: 1033-1042.
- Fleischmann RM, et al: Annals Rheum Dis 2019; 78: 1454-1462.
- Alten R, et al: Patient Prefer Adherence 2016; 10: 2217-2228.
- Current technical information RINVOQ® (upadacitinib). www.swissmedicinfo.ch
- Radner H, et al: Annals of the rheumatic diseases 2014; 73: 114-123.
- Wells G, et al: Ann Rheum Dis 2009; 68(6): 954-960.
FAMILY PRACTICE 2020, 15(9): 28