As more people with type 2 diabetes (T2D) take medication to control blood sugar levels and lose weight, safety concerns have arisen. Real-world evidence from the US Food and Drug Administration’s Adverse Event Reporting System (FA ERS) database now shows a reassuring safety profile for tirzepatide (TZP).
The dual glucose-dependent insulinotropic polypeptide (GIP)/glucagon-like peptide 1 (GLP1) receptor agonist triazepatide has shown high efficacy in lowering blood glucose levels and body weight in people with type 2 diabetes and/or obesity. GLP1 receptor agonists reduce appetite and slow gastric emptying and are therefore increasingly used in the treatment of obesity.
Randomized controlled trials have shown that the safety profile of TZP is similar to that of other GLP1 RAs and is mainly characterized by adverse gastrointestinal events. However, concerns have been raised about a possible association between TZP and diabetic retinopathy, pancreatobiliary disorders and medullary thyroid cancer, but no clear evidence was available yet.
To learn more, Dr. Irene Caruso of the Department of Internal Medicine, Endocrinology, Andrology and Metabolic Diseases at the University of Bari, Italy, and her colleagues searched the FAERS post-marketing surveillance database, which includes reports from manufacturers, patients and healthcare professionals. They wanted to find out whether similar safety concerns have emerged from real-world experience [1].
The researchers retrieved adverse event reports related to TZP that were associated with gastrointestinal disorders, pancreatitis, cholecystitis and cholelithiasis, diabetic retinopathy, and thyroid neoplasms. A reporting odds ratio (ROR) was calculated to assess the disproportionality of reporting certain adverse events associated with TZP compared to other drugs. The analysis was then filtered by reports of age, sex, and the drug designated as the primary suspect. The occurrence of adverse events with TZP was also compared separately with other diabetes treatments only, including insulin, SGLT2 inhibitors, metformin and GLP1-RA (both individually and as a class).
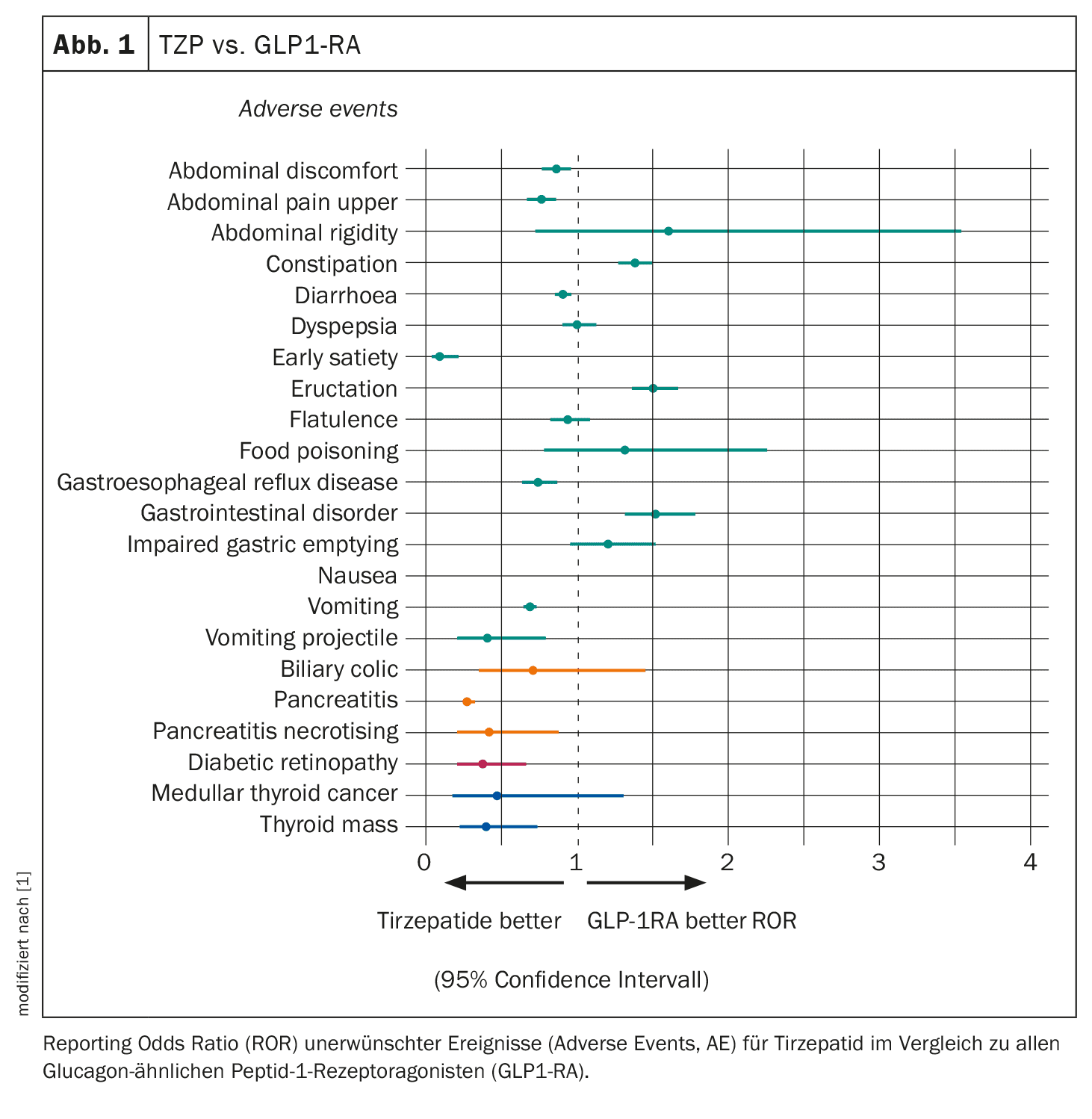
Of the total 20,409 reports on 1432 adverse events, the researchers analyzed 7460 reports relating to 286 selected adverse events (gastrointestinal tract, pancreas, gallbladder, eye and thyroid cancer), 22 of which showed a disproportionality signal.
Lower risk of nausea, but higher risk of constipation
Reports of regurgitation were 30 times more likely with TZP than with other drugs, while nausea, dyspepsia, constipation and pancreatitis were four times more likely with TZP than with all other drugs. However, TZP was associated with a similar risk of gastrointestinal adverse events as other GLP-1RAs, with some differences including a lower risk of nausea and a higher risk of constipation (Fig. 1). As expected, TZP had a higher risk of most gastrointestinal adverse events compared to insulin and SGLT2 inhibitors. No disproportionate reporting of pancreatitis was observed with TZP compared to SGLT2i, but a higher risk was described compared to insulin and a lower risk was reported compared to GLP1-RA.
The researchers also found a disproportionately high report of medullary thyroid cancer with TZP, with a 13-fold higher likelihood compared to all other drugs (based on three events). However, TZP was associated with a similar risk of medullary thyroid cancer as other GLP1-RA and SGLT2 inhibitors and with a higher risk compared to insulin.
Similarly, TZP was more than three times more likely to cause diabetic retinopathy (based on 12 cases) than any other drug. However, the drug had a similar risk to SGLT2 inhibitors and a consistently lower risk than GLP1-RA and insulin. No disproportionate number of adverse events related to the gallbladder and bile ducts were observed, with the exception of an increased risk of biliary colic compared to all other drugs and insulin. Compared to GLP1-RA and SGLT2i, the risk of biliary colic was comparable.
These results showed that tirzepatide has similar gastrointestinal (GI) tolerability compared to the GLP1-RA class, without an increased risk of pancreatitis, diabetic retinopathy and medullary thyroid cancer, Dr. Caruso concluded. The safety profile for tirzepatide is therefore reassuring, albeit with limitations.
Source: Caruso I: The real-world safety profile of tirzepatide: pharmacovigilance analysis of the FDA Adverse Events Reporting System (FAERS) database. Oral Presentation #753, Session SO 063: More combo’s less insulin! EASD, 11.09.2024.
Literature:
- Caruso I, et al: The real-world safety profile of tirzepatide: pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. J Endocrinol Invest 2024; 47: 2671-2678; doi: 10.1007/s40618-024-02441-z.
InFo DIABETOLOGIE & ENDOKRINOLOGIE 2024; 1(4): 18–20 (published on 29.11.24, ahead of print)
HAUSARZT PRAXIS 2024; 19(12): 50–51